Water salinity is of main concern for agriculture in arid and semi-arid areas. Irrigation with brackish water is often the only alternative for farmers in these areas. The C3 water had an average of salinity of 2.10 dS m−1 (oscillating between 2.01 and 2.24 dS m−1), which is viewed as rather good quality water in semi-arid Tunisia. The C4 water had salinity content above 3.46 dS m−1 that reached 7 dS m−1. Na+ and Cl− were the most abundant ions in this water. The effect of both salinity groups was evaluated regarding genotoxicity and root growth of V. faba germinated seedlings.
3.1. Effect of Salinity on Cell Cycle and Growth
Visual effects of high salinity were generally low root and shoot growth and a significant production loss. Being the primary receptor of salt stress and the site of cell cycle and growth, roots act as a barrier against the entry of toxic ions. Cell cycle and cell growth increase cell number and cell volume, respectively, which enables the plant to increase in size. The number of cells in the tissue increases through the mitotic cycle while the volume of individual cells increases through cytoplasmic growth and turgor-driven cell expansion [
8,
9,
19,
21]. As an indicator of cell cycle-linked events, we investigated the mitotic cycle progression by counting dividing cells (MI%). Its resilience was assessed by counting micronucleus (MCN‰) and photographing chromosomal abnormalities. Roots affected by C3 water (varying between EC of 2.01 and 2.24 dS m
−1) had an MI average of 34.0% (16–53%), a low MCN equal to 4.2‰ (2.0–7.5‰), mean root length of 3.38 cm, and FM and DM equal to 0.31 and 0.027 g, respectively. No effects of EC on mitotic cycle progression, MCN formation, as well as growth parameters were observed for C3. Instead, significant positive correlation was found between dividing cell rate and root length (0.518,
p < 0.01)
The increase of salinity (C4) triggered a significant decrease in the number of cell divisions 12.7% (6.3–22.0%) and, inversely, an increase of MCN formation of 7.2‰ (2–19.5‰) compared to C3. Results showed that high EC resulted in negative correlation with MI (r = −0.764,
p < 0.01) while positive correlation was found between EC and MCN (r = 0.561,
p < 0.01). This is interpreted as salinity that damages the cell cycle through a decrease in dividing cell numbers and alteration of chromosome integrity which, consequently, generate MCN. Only cells with normal division phases were counted, which may, in part, explain this decrease. Abundant defective cells in different mitotic phases containing chromosome/chromatid aberrations were excluded. The defective cells generally induced MCN that are formed from acentric chromosome/chromatid fragments or the whole chromosome/chromatid that lag in anaphase and fail to be incorporated in the daughter nucleus. Probably also, this process is affected by nuclear buds attached to the nucleus by a thin nucleoplasm connection [
22].
Cell division affects the root length, as an important indicator of salt stress sensitivity/tolerance. In our case, reduction in root growth was observed for the high-saline water (C4). The largest root length for C4 (3.80 cm) was observed for 3.72 dS m
−1 and the smallest (2.20 cm) for 7 dS m
−1. FM and DM (fresh and dry matter, respectively) decreased, in turn, by 10.5 and 9.6%, respectively, compared to C3 water, despite the short exposure to saltwater (only 48 h). Such reduction in root growth is, in part, the result of decrease in dividing cell number (MI decrease) and the appearance of abnormal cells with MCN. This was confirmed by the negative correlation between MCN and RL (r = −0.519,
p < 0.01), albeit weak and non-significant in case of FM and DM. The effect of salinity on cell production was studied by West et al. who showed that growth decrease of the primary roots of Arabidopsis under salt stress was induced by decrease in cell number and smaller mature cell length [
7]. They emphasized the inhibitor effect of salt on the cyclin dependent kinases’ (CDKs) activities. CDKs are regulatory proteins that control cell division and modulate transcription in response to harmful conditions, by complexing with the cyclins [
23]. In fact, the progression of the cell cycle, which is formed by G1, S, G2, and M (mitosis) phases, is controlled by checkpoints. Cell cycle checkpoints ensure control of cell size and accurate replication and integrity of the chromosomes by preventing cells with damaged or incompletely replicated DNA from entering mitosis and promote the appropriate segregation at mitosis through a control of mitotic spindle [
24]. Checkpoints, CDKs, as well as cyclin activity defects with reduced root growth under salt stress have been well documented [
7,
9,
23] and in part explain the various clastogenic and aneugenic aberrations found under salinity conditions. Chromosomal fragments and bridges are considered as structural abnormalities that may promote smaller MCN size (clastogenic action of salt). Laggings (chromosomes or chromatids) are numerical abnormalities that may induce larger MCN size (aneugenic effect of salt).
The suppression of plant hormone signaling pathways resulting in lower cell cycle activity also results in decreased root elongation. Plant hormones include abscisic acid, auxin, cytokinin, brassinosteroid, gibberellin, and ethylene. These are considered as essential molecules for root growth processes, promoting cell division, cell expansion and elongation, and cell differentiation [
7,
25,
26,
27]. Harmful effects by salt have been attributed to the inhibition of calcium signaling pathways and reactive oxygen species generation causing oxidative damages to nucleic acid bases, by modifying bases and favoring single or double strand breaks in DNA, altering cytosine methylation, and activating programmed cell death (e.g., [
28,
29,
30]).
3.2. Salt Ion Interference with Genome Stability and Growth
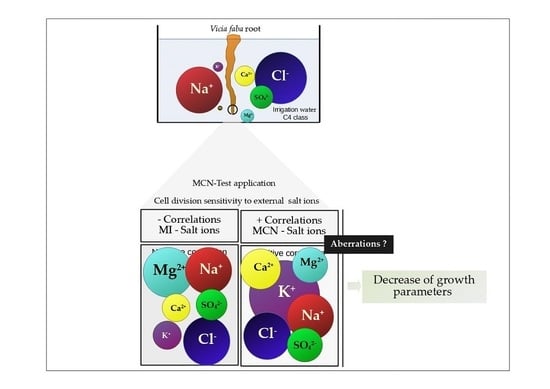
Detrimental effects of salt may lead to toxicity due to specific ions. Specific ion toxicity is usually associated with excessive intake of Na+, Cl−, or other ions, which, once in the plant, disrupt the ion homeostasis mechanisms and damage plant functions. Cell division integrity and root elongation are not excluded from such damage. In this regard, the relationship between, on one hand, genotoxicity, and root growth parameters, and, on the other hand, cations (Na+, Ca2+, Mg2+, and K+) as well as anions (Cl−, SO42−, and HCO3−), was investigated. Mitotic division was negatively influenced by abundant or lower concentration of salt ions in the medium, except for HCO3−. Correlation was strong for Mg2+ (r = −0.827, p < 0.01), Na+ (r = −0.771, p < 0.01), and Cl− (r = −0.805, p < 0.01) and moderate for Ca2+ (r = −0.465, p < 0.05), K+ (r = −0.417, p < 0.05), and SO42− (r = −0.518, p < 0.01). The formation of micronuclei entities was related strongly to K+ (r = 0.915, p < 0.05) and moderately or weakly to other ions. Growth pa-rameters such as root length, in turn, were strongly negatively correlated to the main cations K+ and Ca2+ found at high concentrations in C4 water.
Negative correlation was found between MI and salt ions, and positive correlation for MCN and salt ions. This shows that cations/anions exert mitodepressive effects at higher salt concentrations (C4 compared to C3). It is also a signal of mitosis sensitivity to the ion level in the medium which may, once in the cytosol, interfere with the cell cycle regulatory processes. Among cations that display interference with cell division, Mg
2+ is involved in the formation of mitotic spindle through polymerization and de-polymerization of microtubules (e.g., [
31]). Abraham and Nair studied cytological changes produced in root meristems of
V. faba after exposure to magnesium sulfate (MgSO
4) [
32]. In addition to its ability to induce micronuclei, chromosome breakage, chromosome clumping, achromatic lesions, and lagging chromosomes, Mg
2+ at high concentrations has been found to induce spindle abnormalities leading to the formation of polyploidy and aneuploidy [
32]. Na
+ and Cl
− have been shown to induce genome disorder. Boyko et al. observed drastic increase of the recombination rates in the presence of Cl
− ions, leading to increased numbers of double strand breaks during DNA replication. Na
+ ions, however, had no remarkable effect on the frequency of genomic rearrangements [
33].
In addition to the strong correlation between MI and Mg
2+, Na
+, and Cl
−, moderate effects were observed for Ca
2+, K
+, and SO
42− for C4 water. SO
42− is the primary source of sulfur vital for cell division and protein synthesis and its availability strongly influences plant growth and development as well as crop yield and quality. Short and long-term deprivation of SO
42− or sulfur affects the cell cycle progress while effects of high concentrations of this ion have been less described (e.g., [
34]).
K
+ and Ca
2+ are both needed to promote cell division and growth. Negative correlation was found between these two cations and MI as well as growth parameters for C4 water. The two cations help to maintain the selectivity and integrity of the cell membrane. K
+ is the most abundant inorganic cation in plants, comprising up to 10% of plant dry matter and it is paramount for ensuring optimal plant growth. It is required for proper cell cycle progression during the transition from G1 to S phase, but high concentrations of K
+ can cause gradual decrease in MI and various chromosome abnormalities [
35]. K
+ is also involved in cell elongation, and many other physiological plant functions (e.g., [
36,
37,
38,
39]). When high contents of Na
+ occur, K
+ uptake is disrupted in favor of Na
+ entry. Plants attempt to maintain a high K
+/Na
+ ratio in the cytosol by regulating the activity of K
+ and Na
+ transporters and H pumps that generate the driving force for transport. However, due to the similar radii of K
+ and Na
+ in their hydrated forms, transport proteins are unable to discriminate between these two ions. Increase in the influx of Na
+ under conditions of high external Na
+, induces an alteration of the K
+/Na
+ ratio following the substitution of Na
+ through K
+ transporters or channels [
38,
40].
Ca
2+ is needed for selective uptake and transport of K
+ across membranes. Therefore, salt stress alleviation by Ca
2+ is attributed to the role of this cation to increase K
+ uptake and transport and prevent Na+ binding to the cell wall and plasma membrane [
41]. Ca
2+ appears to participate in the regulation of several events of cell division including nuclear envelope breakdown and reformation, chromatin fiber compaction, and chromosome segregation at anaphase and cytokinesis. It has been reported that, under abiotic stress, the increase of cytosolic concentrations of Ca
2+ in S phase may be a signal of DNA damage and cell cycle regulation by modulating cyclin expression. Ca
2+, at high exogenous concentrations, has been found to delay cell cycle progression and disrupt anaphase at spindle fiber and chromosome movement levels [
42,
43,
44]. This cation is required for various structural functions in the cell wall and membranes as it can form intermolecular linkages [
45] and constitute a second messenger in many biological systems [
46,
47,
48].