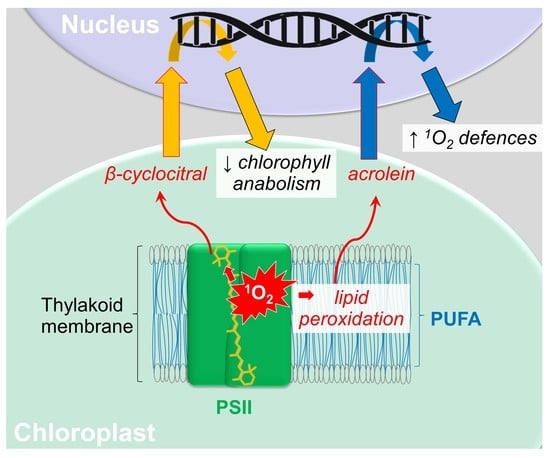
1. Introduction
Photosynthetic organisms often encounter suboptimal conditions, leading to the absorption of excess light energy and ‘high light’ (HL) stress. Therefore, light harvesting must be regulated, requiring acclimation to the current environment [
1]. As part of acclimation, signals from the chloroplast can alter transcription in the nucleus in so-called retrograde signalling [
2,
3]. For example, levels of tetrapyrrole intermediates (chlorophyll precursors) in the chloroplast provide feedback cues to the nucleus during chlorophyll synthesis [
4,
5]. Stress signalling is also partially orchestrated by reactive oxygen species (ROS), including singlet oxygen (
1O
2), which is produced by energy transfer from excited chlorophyll to molecular oxygen in photosystem II (PSII) [
6,
7,
8]. Singlet oxygen oxidises almost anything in its path and the
1O
2 signal leaves the chloroplast in the form of down-stream reaction products. β-cyclocitral, a
1O
2-derived breakdown product of β-carotene, has emerged as an aldehyde electrophile involved in
1O
2 retrograde signalling of
Arabidopsis thaliana [
9]. However, the contribution of β-cyclocitral to
1O
2 signalling in algae is unknown.
Other
1O
2-derived molecules with potent signalling activity include α,β-unsaturated carbonyl derivatives, known as reactive carbonyl/electrophile species (RES). These are produced as a consequence of lipid peroxidation [
10,
11]. Thylakoid membranes are particularly enriched in 1-linolenoyl/2-linolenoyl (di-Cl8:3) found in monogalactosyldiacylglycerol, a polar lipid that improves fluidity for membrane functionality, but is highly prone to peroxidation by
1O
2. One of the most abundant RES produced by lipid peroxidation in chloroplasts due to HL stress is acrolein [
10,
11], which can activate a significant proportion of transcriptional changes that occur in response to
1O
2 [
12,
13]. In
Chlamydomonas reinhardtii, an electrophile response element (ERE)-containing bZIP transcription factor called SOR1 participates in
1O
2 signalling [
12,
13]. SOR1 up-regulates transcription of a large suite of genes, including glutathione transferases (e.g.,
GSTS1) and an isoflavone reductase-like protein (
IRL1), which contribute to RES-associated antioxidant defences [
12,
14,
15].
Although they are potent signalling molecules, RES are also toxic to cells by forming Michael addition adducts with nucleophilic thiolate anions [
16], such as redox-active cysteine residues of proteins. As a thiol, the antioxidant glutathione (GSH), can detoxify RES.
Chlamydomonas reinhardtii rapidly responds to low concentrations of the RES acrolein (≤600 ppm) by increasing GSH contents, but is critically GSH-depleted at higher acrolein concentrations [
13]. Another component of RES detoxification putatively includes glutathione peroxidases (GPX), whereby
GPX5 (also known as GPXh) transcription is strongly up-regulated by sub-lethal levels of
1O
2 [
17,
18] and acrolein [
13]. Moreover, overexpression of GPX5 in
C. reinhardtii can increase tolerance to
1O
2 [
18].
In summary, low levels of ROS/RES can activate signalling pathways implicated in acclimation, whereas an excess ROS/RES load leads to intolerable stress. Thus, stress responses can be distinguished into eustress and distress: Eustress leads to increased stress tolerance via acclimation in which
1O
2 signalling can be involved, and distress leads to loss of viability due to excess stress (e.g., high RES load) beyond a level that can be compensated for by acclimation [
18,
19,
20].
Here, we explored the potential role for β-cyclocitral in 1O2-mediated signalling and inducing 1O2 tolerance in the unicellular model green alga, C. reinhardtii. First, we confirmed that β-cyclocitral could enter cells by measuring increased concentrations of the molecule in treated cells, and observing the concentration-dependent effect on chlorophyll fluorescence. Then, using RNA-Seq analysis, we analysed the transcriptional response of cells to β-cyclocitral and compared this to previously published data of differential gene expression induced by the photosensitizer rose bengal (RB), to reveal if elements of 1O2 signalling were activated by β-cyclocitral. Further experiments investigated if β-cyclocitral can induce 1O2 tolerance, and comparisons are drawn with responses to acrolein, a 1O2-derived signal resulting from lipid peroxidation of the thylakoid membrane.
2. Results
As an aldehyde, β-cyclocitral could be cytotoxic as well as a signalling molecule. Therefore, to assess toxicity, the impact of various concentrations of β-cyclocitral on photosynthesis was probed via chlorophyll fluorescence. The maximum quantum yield of PSII (
Fv/
Fm), which is an often-used health marker of photosynthetic organisms, decreased in both wild types (WT), cell-wall containing WT-4A and cell-wall-less cw15, in response to β-cyclocitral treatment with ≥10 μL/Petri dish (
Figure 1A), corresponding to ≥50,000 ppm atmospheric concentration (see methods for calculation). In contrast, NPQ that is also measured via chlorophyll fluorescence was affected at much lower β-cyclocitral concentrations (
Figure 1B), including at 0.12 μL/Petri dish (
Figure S1), which corresponds to the 600 ppm treatment used for the RNA-Seq analysis. Cellular concentrations of β-cyclocitral before treatment were 0.2 nmol g
−1 fresh weight, and increased 400 fold 2 h after exogenous treatment at 600 ppm (
Figure S2). There was no difference in the influence of β-cyclocitral on NPQ or
Fv/
Fm between WT-4A and cw15 strains (
Figure 1). Furthermore, the reduction of NPQ was also found in the
npq4,
stt7, and
npq4stt7 mutants (
Figure S1), and thus was independent of LHCSR3- and STT7-mediated NPQ, which are the major NPQ mechanisms in
C. reinhardtii [
1,
21]. Therefore, our results are indicative of a direct physical effect of β-cyclocitral on NPQ that occurs at the level of the thylakoid membrane, similar to an uncoupler. To test this, the activity of the pH-dependent violaxanthin cycle was measured in response to HL. Less zeaxanthin accumulated in β-cyclocitral-treated cells (
Figure S1), indicating that the proton gradient was dissipated by β-cyclocitral.
Aldehydes can be detoxified by GSH and associated enzymes, such as GSTS1. Thus, the response of β-cyclocitral on GSH concentrations was also measured up to 1500 ppm (0.3 μL/Petri dish), and after 4 h, no effect was observed (
Figure S3).
Overall, the lack of decrease in
Fv/
Fm and lack of change in GSH concentrations indicated that β-cyclocitral was not a cytotoxic aldehyde at treatment with up to 1500 ppm, a concentration at which the RES acrolein is lethal [
13]. Moreover, the significant impact of β-cyclocitral on NPQ in WT-4A and cw15 showed that this aldehyde could enter the chloroplast of
C. reinhardtii and that the cell wall was not a hindrance to influx. Therefore, we felt confident to be able to assess the signalling properties of β-cyclocitral in exogenously-treated cells.
One of the most
1O
2-responsive genes in
C. reinhardtii is
GPX5, and GPXs likely have a role in mitigating HL stress by detoxifying aldehydes/RES that are produced as a consequence of
1O
2 production [
18]. We screened three mutants over-expressing
GPX5 in the WT-4A background [
18] and found clearly elevated protein levels of GPX5 in
GPXHOX-11 and
GPX5OX-14, relative to WT-4A, in low light (LL)-treated cells (
Figure 2A). This led to the selection of
GPXHOX-11 for further experiments to see how β-cyclocitral, acrolein, and HL influence GPX5 levels, and how elevated levels of GPX5 affect the response to these treatments and subsequent tolerance to
1O
2. Levels of GPX5 increased in WT-4A cells after 4 h of HL, indicating that this treatment induced
1O
2 production. However, 4 h treatments with 600 ppm of β-cyclocitral or 600 ppm of acrolein did not increase GPX5 levels (
Figure 2B). After 4 h of HL, WT cells increased the GSH contents, indicating that cells were under mild oxidative stress, whereas in
GPXHOX-11, no change in the GSH contents occurred, indicating that cells were under less stress. In support of this, after 4 h of HL,
GPXHOX-11 accumulated less aldehydes (propanal and hexanal) and RES (acrolein and 4-hydroxynonenal) than WT-4A, whereas the fold change of β-cyclocitral was equally low in both genotypes (
Figure 3). After a 4 h treatment with 600 ppm of acrolein, both WT and
GPXHOX-11 accumulated GSH (
Figure S3).
A function of
1O
2-related signalling is partly towards increasing tolerance of this ROS [
18]. Therefore, the influence of exogenous β-cyclocitral and acrolein treatments under LL (as potential components of
1O
2-mediated signalling) on the tolerance to
1O
2 was measured. The influence of HL stress, which increases endogenous
1O
2 levels, was also included as pre-treatment before testing
1O
2 tolerance, and all comparisons were made to LL-treated ‘control’ cells. Tolerance to
1O
2 was tested by incubating cells with the photosensitizer RB. Since the amount of
1O
2 that RB produces is dependent on the degree of photoexcitation (i.e., intensity and duration of light treatment) and RB concentration, various treatments were conducted to test cell tolerance. These constituted 4 μM, 7 μM, and 10 μM of RB, either for 10 min at 250 μmol photons m
−2 s
−1 to provide a short
1O
2 shock, or 24 h at 50 μmol photons m
−2 s
−1 to test longer term endurance. The 24 h endurance test was more severe and led to less cell survival of control cells, under which
GPXHOX-11 was significantly less affected than WT-4A (
Figure 4), with
p < 0.05 when comparing genotype as a factor with MANOVA across all RB concentrations. Pre-treatment with HL increased the tolerance of WT-4A to the
1O
2 shock treatment with 7 μM and 10 μM of RB (
Figure 4A), and the 24 h treatment with 4 μM (
Figure 4B), while affecting
GPXHOX-11 less. This resulted in HL-treated WT-4A and
GPXHOX-11 having a similar
1O
2 tolerance (
Figure 4). Relative to control cells, pre-treatment with 600 ppm of β-cyclocitral had no impact on
1O
2 tolerance in either genotype under all treatments (average
p = 0.895), whereas pre-treatment with 600 ppm of acrolein increased the tolerance of both genotypes (average
p = 0.034). In summary, acrolein and HL induced tolerance to severe
1O
2 stress, whereas β-cyclocitral did not significantly affect tolerance.
To investigate if β-cyclocitral could contribute to
1O
2-mediated signalling in
C. reinhardtii, an RNA-Seq analysis of cells treated for 2 h with 600 ppm of β-cyclocitral under LL (
Table S1) was conducted and compared to the transcriptional response of cells treated with the
1O
2-producing photosensitizer RB at 1 μM under LL (data from [
13]). In response to the β-cyclocitral treatment, only six genes were significantly up-regulated, and 57 genes were down-regulated, when considering a fold change of >2 and modified
t-test
p values of <0.01 (
Table S1). Of the genes down-regulated by β-cyclocitral, 18 and 6 were significantly down-regulated and up-regulated, respectively, by RB (
Table S1;
Figure S4). Differentially expressed genes associated with carotenoid metabolism include carotenoid cleavage dioxygenase 8 (
Cre08.g365851), up-regulated five-fold, and β-carotene ketolase (
BKT1), up-regulated two-fold, but not significantly (
Table S1). Of all significantly down-regulated genes, the only ontological group with >1 hit was ‘porphyrin and chlorophyll metabolism’ with 10 hits:
Cre01.g015350 (
POR1),
Cre01.g050950,
Cre02.g085450 (
CPX1),
Cre05.g242000 (
CHLD),
Cre05.g246800 (
GUN4), Cre06
.g306300 (
CHLI1),
Cre07.g325500 (
CHLH1),
Cre09.g396300 (
PPX1),
Cre12.g510050 (
CTH1),
Cre12.g510800 (
CHLI2), and
Cre16.g663900. Collectively, these genes covered several steps of chlorophyll anabolism (
Figure S5), but the overall overlap of differential gene expression with RB-treated cells was low, as shown by a R
2 linear correlation of <0.01 when including all genes, which increases to 0.15, considering only the 63 genes with significantly altered expression (
Figure 5). In comparison, this contrasts with the much tighter relationship between differential gene expression shared between acrolein-treated and RB-treated cells [
13], which has an R
2 linear correlation of 0.34 and 0.70 when considering expression of total genes and only significantly affected genes, respectively (
Figure 5).
3. Discussion
Ten years ago, the discovery that β-cyclocitral in
A. thaliana effects transcription of several genes know to be affected by
1O
2 [
9] made a coherent link between HL-induced
1O
2 production and ROS-associated retrograde signalling. Subsequently, it was found that β-cyclocitral functions up-stream of MBS1 [
22], a zinc finger protein that regulates
1O
2-dependent gene expression, not only in
A. thaliana but also in
C. reinhardtii [
23]. However, the fact of whether β-cyclocitral actually has a role in
1O
2 signalling in alga remained unknown. Since then, other RES (i.e., acrolein), related to lipid peroxidation rather than carotenoid cleavage, emerged as retrograde signals acting in
1O
2-mediated stress acclimation [
13]. Here, we investigated how β-cyclocitral modulates the physiology and transcription in
C. reinhardtii and made comparisons with transcriptional responses of cells to the photosensitizer RB and the RES acrolein.
The very minor effect of β-cyclocitral on
Fv/
Fm showed how tolerant cells were of this molecule. For example, decreases in
Fv/
Fm occurred at >1000 fold concentrations compared to the effects of acrolein (
Figure 1A; [
13]). The concentration of β-cyclocitral even in very light-stressed
C. reinhardtii has never been measured at >1 nmol g
−1 fresh weight [
13,
24], which is below the cellular concentrations after exogenous treatment with 600 ppm (
Figure S2). Therefore, our data supports that, unlike acrolein, β-cyclocitral does not build up to toxic concentrations in light-stressed cells. Despite the insensitivity of
Fv/
Fm to β-cyclocitral, NPQ was affected at much lower concentrations (
Figure 1B and
Figure S1), confirming that β-cyclocitral was able to enter cells, which otherwise may have contributed to tolerance. Inhibition of NPQ in various NPQ mutants indicates that β-cyclocitral directly affected an over-riding NPQ mechanism, such as the requirement of low luminal pH. This was indeed shown by the lower accumulation of zeaxanthin under HL, which is a process requiring a low luminal pH for violaxanthin de-epoxidase activity, in β-cyclocitral-treated cells (
Figure S1). Therefore, we suggest that β-cyclocitral may act as an uncoupler of the thylakoid membrane potential, comparable to the inhibitory activity of structurally similar monoterpene ketones, such as pulegone, on respiration [
25]. The relevance of this observation is that NPQ protects from
1O
2 production under HL [
1,
24], and thus exogenous β-cyclocitral may increase
1O
2 production and may confound observations of β-cyclocitral involvement in
1O
2 signalling under HL. Here, exogenous treatments for the RNA-Seq were conducted under very LL (2 μmol photons m
−2 s
−1), thus unaffected by lowered NPQ.
Defence against
1O
2 requires many enzymes that mitigate lipid peroxidation, including GPX5 [
18]. In mammalian cells, GSH is the typical GPX substrate to break down H
2O
2, whereas in
C. reinhardtii, a thioredoxin is the reductant of GPX5 that has a close association with
1O
2 stress [
26]. In WT cells, HL induced accumulation of GPX5 (
Figure 2B), alongside higher levels of RES, which were attenuated in
GPXHOX-11 (
Figure 3), a
GPX5 over-expressing mutant with elevated GPX5 levels (
Figure 2). This supports that GPX5 has a role in metabolising HL-induced RES production, and explains why HL stress is associated with elevated GPX5 levels [
26,
27].
GPXHOX-11 also possessed significantly elevated tolerance to long-term
1O
2 treatment with the photosensitizer RB (
Figure 4B), in agreement with results from Ledford et al. [
18]. Comparing the influence of pre-treatments on
1O
2 tolerance, acrolein was able to induce tolerance of WT and
GPXHOX-11, whereas β-cyclocitral could not (
Figure 4). Β-cyclocitral did not induce GSH synthesis, whereas acrolein and HL did (
Figure S3), as also previously shown [
13]. Thus, enhanced
1O
2 tolerance can be partially attributed to elevated GSH and GPX5 levels.
A molecule involved in stress signalling would be expected to increase in concentration in response to the relevant stress. The amounts of β-cyclocitral in
C. reinhardtii were ≤1 nmol g
−1 fresh weight (
Figure S2), and not affected by HL stress (
Figures S2 and
Figure 3), also in agreement with previous data [
13,
24]. In comparison, levels of RES increased in HL-stressed cells, similar to after treatment with RB [
13], supporting that
1O
2 production under HL is involved in RES production, but hardly with β-cyclocitral production. Acrolein has received attention in the field of redox biology for its high electrophilic nature and high endogenous levels of >5 nmol g
−1 fresh weight in stressed plants and algae alike [
13,
28,
29]. Previously, we showed that exogenous acrolein treatments induce a ‘eustress’ (i.e., acclimation) response by up-regulating thiol-disulfide-dependent defence mechanisms required for tolerating
1O
2 [
13]. Of note, around half of global gene expression (up and down) occurring in response to 600 ppm of acrolein, the dose that induced a eustress response with highest tolerance to
1O
2, was shared with the gene regulation in response to RB [
13]. While we did not find a similar transcriptional response to β-cyclocitral (
Figure 5), in line with a lack of inducing
1O
2 tolerance (
Figure 4), there was evidence that β-cyclocitral may have some signalling properties in C
. reinhardtii. Collectively down-regulated genes (
Table S1) covered many steps of chlorophyll anabolism (
Figure S5), such as porphobilinogen deaminase/HemC (
Cre16.g663900.t1.2), coproporphyrinogen III oxidase (
CPX1), and protoporphyrinogen oxidase (
PPX1), which are involved in early steps of porphyrin synthesis, as well as genes coding for proteins that insert Mg
2+ into protoporphyrin, including tetrapyrrole-binding protein (
GUN4) and magnesium chelatase (
CHLI1,
CHLI2,
CHLD,
CHLH1) to form Mg-protoporphyrin IX (MgP), the first dedicated intermediate of the chlorophyll branch. In
C. reinhardtii,
CHLI2 seems to be redundant to
CHLI1 [
30]. Gene expression associated with later steps of chlorophyll synthesis, such as copper target 1 protein (
CTH1) and light-dependent protochlorophyllide reductase (
POR1) were also down-regulated by β-cyclocitral. In
A. thaliana, β-cyclocitral also down-regulated expression of
CHLI2 and a few other genes involved in porphyrin/chlorophyll biosynthesis, such as
PORB,
CHLM and
HEME1 alongside an up-regulation of
CLH1 and
CLH2 involved in chlorophyll catabolism (Ramel et al., 2102), indicating a conserved signalling role for β-cyclocitral in decreasing chlorophyll contents, which existed before the evolution of vascular plants. Chlorophyll synthesis needs to be tightly regulated because MgP and protochlorophyllide are, similar to free chlorophyll, highly efficient photosensitizers [
6]. MgP provides feedback on chlorophyll synthesis by repressing nuclear transcription in a signalling pathway that requires GUN4 [
4]. There are contrasting reports on whether the GUN4-MgP complex produces more or less
1O
2 than MgP alone, and if
1O
2 is a component of the retrograde signal [
31,
32].
In summary, in C. reinhardtii, β-cyclocitral does not seem to have a role in 1O2 signalling or inducing 1O2 tolerance. Nonetheless, the influence of carotenoid cleavage products on chlorophyll synthesis and how 1O2 integrates into the retrograde signalling of this pathway warrants further investigation.