Citrus clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury: Insights into the PI3K/AKT Pathway
Abstract
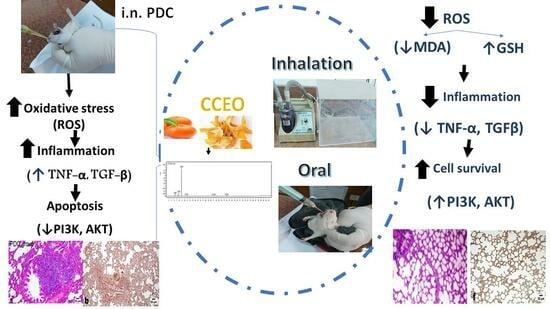
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Plant Materials
2.3. Animals
2.4. Extraction
2.5. Gas Chromatography–Mass Spectrometry Analysis (GC/MS)
2.6. Experimental Design
2.7. Biochemical Determination
2.8. Real-Time Polymerase Chain Reaction (PCR) Quantification of Protein Kinase B (Akt)
2.9. Histopathological and Immunohistochemical Evaluations
2.10. Statistical Analysis
3. Results
3.1. Chemical Profile of CCEO
3.2. Biological Results
3.2.1. LD50 Result
Assessment of MDA and GSH Lung Contents
Assessment of TNF-α and TGF-β Lung Contents
Assessment of PI3K Lung Content
AKT Gene Expression Assessment
Histopathological and Immunohistochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Hegazy, R.; Hassan, A. Intranasal Chromium Induces Acute Brain and Lung Injuries in Rats: Assessment of Different Potential Hazardous Effects of Environmental and Occupational Exposure to Chromium and Introduction of a Novel Pharmacological and Toxicological Animal Model. PLoS ONE 2016, 11, e0168688. [Google Scholar] [CrossRef]
- Zhao, M.; Li, C.; Shen, F.; Wang, M.; Jia, N.; Wang, C. Naringenin Ameliorates LPS-Induced Acute Lung Injury through Its Anti-Oxidative and Anti-Inflammatory Activity and by Inhibition of the PI3K/AKT Pathway. Exp. Ther. Med. 2017, 14, 2228–2234. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; e Silva, C.P.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Asaad, G.F.; Abdelhameed, M.F.; El Raey, M.A.; Roshdy, W.H.; Elgamal, A.M.; Moemen, Y.S. Citrus Clementine Peels Essential Oil Exhibited Anti-SARS-CoV-2 and Its Modulatory Effect against Cytokine Storm: Evidence from in Vitro and in Silico Studies. Egypt. J. Chem. 2022, 65, 419–427. [Google Scholar] [CrossRef]
- Sorino, C.; Negri, S.; Spanevello, A.; Visca, D.; Scichilone, N. Inhalation Therapy Devices for the Treatment of Obstructive Lung Diseases: The History of Inhalers towards the Ideal Inhaler. Eur. J. Intern. Med. 2020, 75, 15–18. [Google Scholar] [CrossRef]
- Alipour, S.; Mahmoudi, L.; Ahmadi, F. Pulmonary Drug Delivery: An Effective and Convenient Delivery Route to Combat COVID-19. Drug Deliv. Transl. Res. 2023, 13, 705–715. [Google Scholar] [CrossRef]
- Alqahtani, A.; Abdelhameed, M.F.; Abdou, R.; Ibrahim, A.M.; Dawoud, M.; Alasmari, S.M.; El Raey, M.A.; Attia, H.G. Mechanistic Action of Linalyl Acetate: Acyclic Monoterpene Isolated from Bitter Orange Leaf as Anti-Inflammatory, Analgesic, Antipyretic Agent: Role of TNF-α, IL1β, PGE2, and COX-2. Ind. Crops Prod. 2023, 203, 117131. [Google Scholar] [CrossRef]
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus Sinensis Essential Oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12, 246. [Google Scholar] [CrossRef]
- El-Fadaly, A.A.; Younis, I.Y.; Abdelhameed, M.F.; Ahmed, Y.H.; Ragab, T.I.M.; El Gendy, A.E.-N.G.; Farag, M.A.; Elshamy, A.I.; Elgamal, A.M. Protective Action Mechanisms of Launaea Mucronata Extract and Its Nano-Formulation against Nephrotoxicity in Rats as Revealed via Biochemical, Histopathological, and UPLC-QTOF–MS/MS Analyses. Metabolites 2023, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- KS Suvarna, C.; Layton, J.B. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Expert Consult: Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Toya, T.; Fukuda, K.; Kohyama, N.; Kyono, H.; Arito, H. Hexavalent Chromium Responsible for Lung Lesions Induced by Intratracheal Instillation of Chromium Fumes in Rats. Ind. Health 1999, 37, 36–46. [Google Scholar] [CrossRef]
- El-Daly, S.M.; El-Bana, M.A.; Abd El-Rahman, S.S.; Latif, Y.A.; Medhat, D. Dynamic Expression of H19 and MALAT1 and Their Correlation with Tumor Progression Biomarkers in a Multistage Hepatocarcinogenesis Model. Cell Biochem. Funct. 2023, 41, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Alhumaydhi, F.A.; Aljohani, A.S.M.; Elsharkawy, E.R. UPLC/ESI-MS Phytochemical Screening of Deverra Tortuosa Haematological and Histopathological Studies and Streptozotocin-Induced Diabetes in Rat. Evid.-Based Complement. Altern. Med. 2021, 2021, 4718854. [Google Scholar] [CrossRef]
- Dugo, G.; Cotroneo, A.; Bonaccorsi, I.L.; Trozzi, A. Composition of the Volatile Fraction of Citrus Peel Oils. In Citrus Oils. Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity; CRC Press Tayolor and Francis Group: Boca Raton, FL, USA, 2011; pp. 1–161. ISBN 1439800286. [Google Scholar]
- İsmail Kirbaşlar, Ş.; Gök, A.; Gülay Kirbaşlar, F.; Tepe, S. Volatiles in Turkish Clementine (Citrus Clementina Hort.) Peel. J. Essent. Oil Res. 2012, 24, 153–157. [Google Scholar] [CrossRef]
- Barboni, T.; Paolini, J.; Tomi, P.; Luro, F.; Muselli, A.; Costa, J. Characterization and Comparison of Volatile Constituents of Juice and Peel from Clementine, Mandarin and Their Hybrids. Nat. Prod. Commun. 2011, 6, 1495-8. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Silva, A.S.; Carreiró, F.; Loizzo, M.R. Health-Promoting Properties and Potential Application in the Food Industry of Citrus Medica L. and Citrus × Clementina Hort. Ex Tan. Essential Oils and Their Main Constituents. Plants 2023, 12, 991. [Google Scholar] [CrossRef]
- Thi Nguyen, T.-T.; Thi Tran, T.-T.; Hua, T.-M.; Diep, T.-T.; Chau, D.-K.N.; Duus, F.; Le, T.N. Investigation of Peel and Leaf Essential Oils of Citrus Clementina Hort. Ex Tan. Growing in the South of Vietnam. J. Essent. Oil Res. 2016, 28, 96–103. [Google Scholar] [CrossRef]
- Saleem, M.; Durani, A.I.; Asari, A.; Ahmed, M.; Ahmad, M.; Yousaf, N.; Muddassar, M. Investigation of Antioxidant and Antibacterial Effects of Citrus Fruits Peels Extracts Using Different Extracting Agents: Phytochemical Analysis with in Silico Studies. Heliyon 2023, 9, e15433. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules 2023, 28, 1624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Xiang, D.; Ju, L.; Shen, D.; Wang, X.; Wang, Y. Friend or Foe? The Roles of Antioxidants in Acute Lung Injury. Antioxidants 2021, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W. Enteral Omega-3 Fatty Acid, γ-Linolenic Acid, and Antioxidant Supplementation in Acute Lung Injury. JAMA 2011, 306, 1574. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant Supplements for Prevention of Mortality in Healthy Participants and Patients with Various Diseases. Cochrane Database Syst. Rev. 2012, 2012, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated Limonene: A Pleasant Lemon-Like Aroma with Promising Application in the Agri-Food Industry. A Review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.D.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes Modulating Cytokines—A Review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Tao, Y.; Chen, S.; Luo, H.; Li, X.; Qu, S.; Chen, K.; Zeng, C. Rosmarinic Acid Ameliorates Pulmonary Ischemia/Reperfusion Injury by Activating the PI3K/Akt Signaling Pathway. Front. Pharmacol. 2022, 13, 860944. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Wang, L.; Du, F.; Zhou, X.; Song, Q.; Zhao, J.; Fang, R. Ginsenoside Rg3 Attenuates Lipopolysaccharide-Induced Acute Lung Injury via MerTK-Dependent Activation of the PI3K/AKT/MTOR Pathway. Front. Pharmacol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lin, B.; Gao, Y.; Lei, X.; Wang, X.; Li, Y.; Li, T. Genipin Attenuates Mitochondrial-Dependent Apoptosis, Endoplasmic Reticulum Stress, and Inflammation via the PI3K/AKT Pathway in Acute Lung Injury. Int. Immunopharmacol. 2019, 76, 105842. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, N.; Ma, Z.; Wang, Y.; Yuan, Y.; Zhong, Z.; Hong, Y.; Zhao, M. Lipoxin A4 Protects against Paraquat-induced Acute Lung Injury by Inhibiting the TLR4/MyD88-mediated Activation of the NF-κB and PI3K/AKT Pathways. Int. J. Mol. Med. 2021, 47, 86. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Jin, Y.; Zhang, Y.; Li, S.; Cui, J.; He, H.; Guo, L.; Yang, F.; Liu, H. Inhibition of Oncogenic Src Ameliorates Silica-Induced Pulmonary Fibrosis via PI3K/AKT Pathway. Int. J. Mol. Sci. 2023, 24, 774. [Google Scholar] [CrossRef]
- Du, X.; Feng, W.; Zhang, S.; Lin, X.; Song, L.; Shen, N.; Yang, X. Salidroside Attenuates LPS-Induced Inflammatory Activation in Young Rats with Acute Lung Injury via PI3K/Akt Signaling Pathway. Cell. Mol. Biol. 2023, 69, 124–128. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Tang, H.; Hu, X.; Hubert, S.M.; Li, Q.; Su, D.; Xu, H.; Fan, Y.; Yu, X.; et al. Activation of PI3K/AKT Pathway Is a Potential Mechanism of Treatment Resistance in Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 526–539. [Google Scholar] [CrossRef]
- Iksen; Pothongsrisit, S.; Pongrakhananon, V. Targeting the PI3K/AKT/MTOR Signaling Pathway in Lung Cancer: An Update Regarding Potential Drugs and Natural Products. Molecules 2021, 26, 4100. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Asha, K.; Sanicas, M.; Asi, A.; Arya, U.M.; Kumar, B. PI3K/Akt/Nrf2 Mediated Cellular Signaling and Virus-Host Interactions: Latest Updates on the Potential Therapeutic Management of SARS-CoV-2 Infection. Front. Mol. Biosci. 2023, 10, 1158133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, H.; Cai, H.; Chen, G.; Chen, H.; Ruan, W. Progression of the PI3K/Akt Signaling Pathway in Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 2023, 14, 1238782. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Brognard, J.; Clark, A.S.; Linnoila, I.R.; Yang, X.; Swain, S.M.; Harris, C.; Belinsky, S.; Dennis, P.A. Rapid Akt Activation by Nicotine and a Tobacco Carcinogen Modulates the Phenotype of Normal Human Airway Epithelial Cells. J. Clin. Investig. 2003, 111, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.R.; de Carvalho, F.O.; Menezes dos Santos, D.; da Silva, E.A.P.; Silva, É.R.; Shanmugam, S.; Heimfarth, L.; Nunes, P.S.; de Oliveira e Silva, A.M.; Araújo, A.A.d.S.; et al. Inhaled D-Limonene Minimizes Acute Lung Injury and Reduces Oxidative Stress Induced by Smoke in Rats. Phytomed. Plus 2022, 2, 100308. [Google Scholar] [CrossRef]
- Levet, V.; Merlos, R.; Rosière, R.; Amighi, K.; Wauthoz, N. Platinum Pharmacokinetics in Mice Following Inhalation of Cisplatin Dry Powders with Different Release and Lung Retention Properties. Int. J. Pharm. 2017, 517, 359–372. [Google Scholar] [CrossRef]
- Chen, C.; Sheng, Y.; Hu, Y.; Sun, J.; Li, W.; Feng, H.; Tang, L. Determination of d-limonene in Mice Plasma and Tissues by a New GC–MS/MS Method: Comparison of the Pharmacokinetics and Tissue Distribution by Oral and Inhalation Administration in Mice. Biomed. Chromatogr. 2019, 33, e4530. [Google Scholar] [CrossRef]










| Peak | Retention Index (RI) | Area% | Identified Compounds | Chemical Class | |
|---|---|---|---|---|---|
| Reported | Literature | ||||
| 1 | 928 | 932 | 2.48 | α-Pinene | Monoterpene |
| 2 | 959 | 969 | 1.77 | Sabinene | Monoterpene |
| 3 | 985 | 990 | 3.94 | β-Myrcene | Monoterpene |
| 4 | 1019 | 1024 | 88.84 | D-Limonene | Monoterpene |
| 5 | 1094 | 1095 | 0.86 | Linalool | Oxygenated monoterpene |
| 6 | 1434 | 1432 | 0.6 | β-Copaene | Sesquiterpene |
| 7 | 1532 | 1529 | 0.64 | β-Cadinene | Sesquiterpene |
| 8 | 1699 | 1997 | 0.87 | β-Sinensal | Oxygenated Sesquiterpene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, H.G.; El-Morshedy, S.M.; Nagy, A.M.; Ibrahim, A.M.; Aleraky, M.; Abdelrahman, S.S.; Osman, S.M.; Alasmari, S.M.; El Raey, M.A.; Abdelhameed, M.F. Citrus clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury: Insights into the PI3K/AKT Pathway. Metabolites 2024, 14, 68. https://doi.org/10.3390/metabo14010068
Attia HG, El-Morshedy SM, Nagy AM, Ibrahim AM, Aleraky M, Abdelrahman SS, Osman SM, Alasmari SM, El Raey MA, Abdelhameed MF. Citrus clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury: Insights into the PI3K/AKT Pathway. Metabolites. 2024; 14(1):68. https://doi.org/10.3390/metabo14010068
Chicago/Turabian StyleAttia, Hany G., Suzan M. El-Morshedy, Ahmed M. Nagy, Ammar M. Ibrahim, Mohamed Aleraky, Sahar S. Abdelrahman, Samir M. Osman, Saeed M. Alasmari, Mohamed A. El Raey, and Mohamed F. Abdelhameed. 2024. "Citrus clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury: Insights into the PI3K/AKT Pathway" Metabolites 14, no. 1: 68. https://doi.org/10.3390/metabo14010068