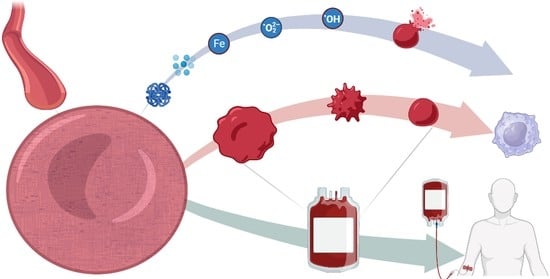
Red Blood Cell Metabolism In Vivo and In Vitro
Abstract
:1. Red Blood Cell Metabolism: The Central Role of Oxygen
2. RBC Metabolism beyond Glycolysis
3. RBC Metabolism and Blood Storage for Clinical Transfusion Purposes
4. All Blood Units Are Created Equal, but Some Blood Units Are More Equal than Others
Author Contributions
Funding
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Piovesan, A.; Facchin, F.; Beraudi, A.; Casadei, R.; Frabetti, F.; Vitale, L.; Pelleri, M.C.; Tassani, S.; Piva, F.; et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Reisz, J.A.; Xia, Y.; Zimring, J.C.; D’Alessandro, A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteom. 2018, 15, 855–864. [Google Scholar] [CrossRef]
- Klei, T.R.L.; Meinderts, S.M.; van den Berg, T.K.; van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, a011601. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Righetti, P.G.; Zolla, L. The red blood cell proteome and interactome: An update. J. Proteome Res. 2010, 9, 144–163. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef]
- Kanias, T.; Acker, J.P. Biopreservation of red blood cells—The struggle with hemoglobin oxidation. Febs J. 2010, 277, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Wither, M.; Dzieciatkowska, M.; Nemkov, T.; Strop, P.; D’Alessandro, A.; Hansen, K.C. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion 2016, 56, 421–426. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Blasi, B.; D’Amici, G.M.; Marrocco, C.; Zolla, L. Red blood cell subpopulations in freshly drawn blood: Application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 2013, 11, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dreischer, P.; Duszenko, M.; Stein, J.; Wieder, T. Eryptosis: Programmed Death of Nucleus-Free, Iron-Filled Blood Cells. Cells 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Ghatge, M.S.; Safo, M.K. Hemoglobin: Structure, Function and Allostery. Subcell. Biochem. 2020, 94, 345–382. [Google Scholar] [CrossRef]
- Yuan, Y.; Tam, M.F.; Simplaceanu, V.; Ho, C. New look at hemoglobin allostery. Chem. Rev. 2015, 115, 1702–1724. [Google Scholar] [CrossRef]
- Donovan, K.; Meli, A.; Cendali, F.; Park, K.C.; Cardigan, R.; Stanworth, S.; McKechnie, S.; D’Alessandro, A.; Smethurst, P.A.; Swietach, P. Stored blood has compromised oxygen unloading kinetics that can be normalized with rejuvenation and predicted from corpuscular side-scatter. Haematologica 2022, 107, 298–302. [Google Scholar] [CrossRef]
- Gatto, C.; Milanick, M. Red blood cell Na pump: Insights from species differences. Blood Cells Mol. Dis. 2009, 42, 192–200. [Google Scholar] [CrossRef]
- Longo, V.; Marrocco, C.; Zolla, L.; Rinalducci, S. Label-free quantitation of phosphopeptide changes in erythrocyte membranes: Towards molecular mechanisms underlying deformability alterations in stored red blood cells. Haematologica 2014, 99, e122–e125. [Google Scholar] [CrossRef]
- Xu, P.; Chen, C.; Zhang, Y.; Dzieciatkowska, M.; Brown, B.C.; Zhang, W.; Xie, T.; Abdulmalik, O.; Song, A.; Tong, C.; et al. Erythrocyte transglutaminase-2 combats hypoxia and chronic kidney disease by promoting oxygen delivery and carnitine homeostasis. Cell Metab. 2022, 34, 299–316.e6. [Google Scholar] [CrossRef]
- Betz, T.; Lenz, M.; Joanny, J.-F.; Sykes, C. ATP-dependent mechanics of red blood cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15320–15325. [Google Scholar] [CrossRef]
- Gokhin, D.S.; Fowler, V.M. Feisty filaments: Actin dynamics in the red blood cell membrane skeleton. Curr. Opin. Hematol. 2016, 23, 206–214. [Google Scholar] [CrossRef]
- Wagner, G.M.; Chiu, D.T.; Yee, M.C.; Lubin, B.H. Red cell vesiculation—A common membrane physiologic event. J. Lab. Clin. Med. 1986, 108, 315–324. [Google Scholar]
- Arashiki, N.; Takakuwa, Y.; Mohandas, N.; Hale, J.; Yoshida, K.; Ogura, H.; Utsugisawa, T.; Ohga, S.; Miyano, S.; Ogawa, S.; et al. ATP11C is a major flippase in human erythrocytes and its defect causes congenital hemolytic anemia. Haematologica 2016, 101, 559–565. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Tzounakas, V.L.; Arvaniti, V.Z.; Dzieciatkowska, M.; Stamoulis, K.; Lekka, M.E.; Papassideri, I.S.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Red Blood Cell Proteasome in Beta-Thalassemia Trait: Topology of Activity and Networking in Blood Bank Conditions. Membranes 2021, 11, 716. [Google Scholar] [CrossRef]
- Song, A.; Wen, A.Q.; Wen, Y.E.; Dzieciatkowska, M.; Kellems, R.E.; Juneja, H.S.; D’Alessandro, A.; Xia, Y. p97 dysfunction underlies a loss of quality control of damaged membrane proteins and promotes oxidative stress and sickling in sickle cell disease. FASEB J. 2022, 36, e22246. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Dzieciatkowska, M.; Anastasiadi, A.T.; Karadimas, D.G.; Vergaki, A.; Siourounis, P.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; D’Alessandro, A.; et al. Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus. 2022, 20, 27–39. [Google Scholar] [CrossRef]
- van Wijk, R.; van Solinge, W.W. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Sun, K.; Liu, H.; Song, A.; Monte, A.A.; Subudhi, A.W.; Lovering, A.T.; Dvorkin, D.; Julian, C.G.; et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res. 2016, 15, 3883–3895. [Google Scholar] [CrossRef]
- Reisz, J.A.; Slaughter, A.L.; Culp-Hill, R.; Moore, E.E.; Silliman, C.C.; Fragoso, M.; Peltz, E.D.; Hansen, K.C.; Banerjee, A.; D’Alessandro, A. Red blood cells in hemorrhagic shock: A critical role for glutaminolysis in fueling alanine transamination in rats. Blood Adv. 2017, 1, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Hansen, K.C.; Eisenmesser, E.Z.; Zimring, J.C. Protect, repair, destroy or sacrifice: A role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus. 2019, 17, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.M.; Stefely, J.A.; Veling, M.T.; van ‘t Erve, T.J.; Wagner, B.A.; Raife, T.J.; Buettner, G.R. Red blood cells contain enzymatically active GPx4 whose abundance anticorrelates with hemolysis during blood bank storage. Redox Biol. 2021, 46, 102073. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; D’Amici, G.M.; Blasi, B.; Vaglio, S.; Grazzini, G.; Zolla, L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 2011, 51, 1439–1449. [Google Scholar] [CrossRef]
- Paukovich, N.; Xue, M.; Elder, J.R.; Redzic, J.S.; Blue, A.; Pike, H.; Miller, B.G.; Pitts, T.M.; Pollock, D.D.; Hansen, K.; et al. Biliverdin Reductase B Dynamics Are Coupled to Coenzyme Binding. J. Mol. Biol. 2018, 430, 3234–3250. [Google Scholar] [CrossRef]
- Corti, A.; Casini, A.F.; Pompella, A. Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Arch. Biochem. Biophys. 2010, 500, 107–115. [Google Scholar] [CrossRef]
- Pey, A.L.; Megarity, C.F.; Timson, D.J. NAD(P)H quinone oxidoreductase (NQO1): An enzyme which needs just enough mobility, in just the right places. Biosci. Rep. 2019, 39, BSR20180459. [Google Scholar] [CrossRef]
- Fenk, S.; Melnikova, E.V.; Anashkina, A.A.; Poluektov, Y.M.; Zaripov, P.I.; Mitkevich, V.A.; Tkachev, Y.V.; Kaestner, L.; Minetti, G.; Mairbäurl, H.; et al. Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability. Redox Biol. 2022, 58, 102535. [Google Scholar] [CrossRef]
- Harper, V.M.; Oh, J.Y.; Stapley, R.; Marques, M.B.; Wilson, L.; Barnes, S.; Sun, C.W.; Townes, T.; Patel, R.P. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid. Redox Signal. 2015, 22, 294–307. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef]
- Katabami, K.; Hayakawa, M.; Gando, S. Severe Methemoglobinemia due to Sodium Nitrite Poisoning. Case Rep. Emerg. Med. 2016, 2016, 9013816. [Google Scholar] [CrossRef]
- Parashar, A.; Jacob, V.D.; Gideon, D.A.; Manoj, K.M. Hemoglobin catalyzes ATP-synthesis in human erythrocytes: A murburn model. J. Biomol. Struct. Dyn. 2022, 40, 8783–8795. [Google Scholar] [CrossRef]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic. Biol. Med. 2016, 96, 152–165. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Data on how several physiological parameters of stored red blood cells are similar in glucose 6-phosphate dehydrogenase deficient and sufficient donors. Data Brief. 2016, 8, 618–627. [Google Scholar] [CrossRef]
- Francis, R.O.; D’Alessandro, A.; Eisenberger, A.; Soffing, M.; Yeh, R.; Coronel, E.; Sheikh, A.; Rapido, F.; La Carpia, F.; Reisz, J.A.; et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J. Clin. Investig. 2020, 130, 2270–2285. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Chen, X.; Wang, Q.; Ling, L.; Xu, Y. Glucose-6-phosphate dehydrogenase deficiency in the Han Chinese population: Molecular characterization and genotype-phenotype association throughout an activity distribution. Sci. Rep. 2020, 10, 17106. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Fu, X.; Kanias, T.; Reisz, J.A.; Culp-Hill, R.; Guo, Y.; Gladwin, M.T.; Page, G.; Kleinman, S.; Lanteri, M.; et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 2021, 106, 1290–1302. [Google Scholar] [CrossRef]
- Page, G.P.; Kanias, T.; Guo, Y.J.; Lanteri, M.C.; Zhang, X.; Mast, A.E.; Cable, R.G.; Spencer, B.R.; Kiss, J.E.; Fang, F.; et al. Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J. Clin. Investig. 2021, 131, e146077. [Google Scholar] [CrossRef]
- Archer, N.M.; Petersen, N.; Clark, M.A.; Buckee, C.O.; Childs, L.M.; Duraisingh, M.T. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, 7350–7355. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Stefanoni, D.; Cendali, F.; Bertolone, L.; Gamboni, F.; Dzieciatkowska, M.; Rousakis, P.; Vergaki, A.; Soulakis, V.; et al. β-thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 2022, 107, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Mbanefo, E.C.; Ahmed, A.M.; Titouna, A.; Elmaraezy, A.; Trang, N.T.; Phuoc Long, N.; Hoang Anh, N.; Diem Nghi, T.; The Hung, B.; Van Hieu, M.; et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 45963. [Google Scholar] [CrossRef]
- Reisz, J.A.; Wither, M.J.; Dzieciatkowska, M.; Nemkov, T.; Issaian, A.; Yoshida, T.; Dunham, A.J.; Hill, R.C.; Hansen, K.C.; D’Alessandro, A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016, 128, e32–e42. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, C.; Manuel, A.M.; Sharafi, M.; Aboushousha, R.; Qian, X.; Erickson, C.; MacPherson, M.; Chan, G.; Adcock, I.M.; ZounematKermani, N.; et al. Glutathione-S-transferase P promotes glycolysis in asthma in association with oxidation of pyruvate kinase M2. Redox Biol. 2021, 47, 102160. [Google Scholar] [CrossRef] [PubMed]
- Aboushousha, R.; Elko, E.; Chia, S.B.; Manuel, A.M.; van de Wetering, C.; van der Velden, J.; MacPherson, M.; Erickson, C.; Reisz, J.A.; D’Alessandro, A.; et al. Glutathionylation chemistry promotes interleukin-1 beta-mediated glycolytic reprogramming and pro-inflammatory signaling in lung epithelial cells. Faseb J. 2021, 35, e21525. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.A.; Campanella, M.E.; Markley, J.L.; Low, P.S. Role of band 3 in regulating metabolic flux of red blood cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18515–18520. [Google Scholar] [CrossRef]
- Chu, H.; Low, P.S. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem. J. 2006, 400, 143–151. [Google Scholar] [CrossRef]
- Messana, I.; Ferroni, L.; Misiti, F.; Girelli, G.; Pupella, S.; Castagnola, M.; Zappacosta, B.; Giardina, B. Blood bank conditions and RBCs: The progressive loss of metabolic modulation. Transfusion 2000, 40, 353–360. [Google Scholar] [CrossRef]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 2021, 106, 2971–2985. [Google Scholar] [CrossRef]
- Rogers, S.C.; Ge, X.; Brummet, M.; Lin, X.; Timm, D.D.; d’Avignon, A.; Garbow, J.R.; Kao, J.; Prakash, J.; Issaian, A.; et al. Quantifying dynamic range in red blood cell energetics: Evidence of progressive energy failure during storage. Transfusion 2021, 61, 1586–1599. [Google Scholar] [CrossRef]
- Wiback, S.J.; Palsson, B.O. Extreme pathway analysis of human red blood cell metabolism. Biophys. J. 2002, 83, 808–818. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Pajerowski, J.D.; Jamshidi, N.; Palsson, B.O.; Edwards, J.S. Description and Analysis of Metabolic Connectivity and Dynamics in the Human Red Blood Cell. Biophys. J. 2002, 83, 646–662. [Google Scholar] [CrossRef]
- Nakayama, Y.; Kinoshita, A.; Tomita, M. Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor. Biol. Med. Model. 2005, 2, 18. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Dzieciatkowska, M.; Nemkov, T.; Hansen, K.C. Red blood cell proteomics update: Is there more to discover? Blood Transfus. 2017, 15, 182–187. [Google Scholar] [CrossRef]
- Gautier, E.F.; Leduc, M.; Cochet, S.; Bailly, K.; Lacombe, C.; Mohandas, N.; Guillonneau, F.; El Nemer, W.; Mayeux, P. Absolute proteome quantification of highly purified populations of circulating reticulocytes and mature erythrocytes. Blood Adv. 2018, 2, 2646–2657. [Google Scholar] [CrossRef]
- Pasini, E.M.; Kirkegaard, M.; Mortensen, P.; Lutz, H.U.; Thomas, A.W.; Mann, M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood 2006, 108, 791–801. [Google Scholar] [CrossRef]
- Sae-Lee, W.; McCafferty, C.L.; Verbeke, E.J.; Havugimana, P.C.; Papoulas, O.; McWhite, C.D.; Houser, J.R.; Vanuytsel, K.; Murphy, G.J.; Drew, K.; et al. The protein organization of a red blood cell. Cell Rep. 2022, 40, 111103. [Google Scholar] [CrossRef]
- Wilson, M.C.; Trakarnsanga, K.; Heesom, K.J.; Cogan, N.; Green, C.; Toye, A.M.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Comparison of the Proteome of Adult and Cord Erythroid Cells, and Changes in the Proteome Following Reticulocyte Maturation. Mol. Cell. Proteom. 2016, 15, 1938–1946. [Google Scholar] [CrossRef]
- Goodman, S.R.; Daescu, O.; Kakhniashvili, D.G.; Zivanic, M. The proteomics and interactomics of human erythrocytes. Exp. Biol. Med. 2013, 238, 509–518. [Google Scholar] [CrossRef]
- Paglia, G.; D’Alessandro, A.; Rolfsson, O.; Sigurjonsson, O.E.; Bordbar, A.; Palsson, S.; Nemkov, T.; Hansen, K.C.; Gudmundsson, S.; Palsson, B.O. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016, 128, e43–e50. [Google Scholar] [CrossRef]
- Bordbar, A.; McCloskey, D.; Zielinski, D.C.; Sonnenschein, N.; Jamshidi, N.; Palsson, B.O. Personalized Whole-Cell Kinetic Models of Metabolism for Discovery in Genomics and Pharmacodynamics. Cell Syst. 2015, 1, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, A.; Jamshidi, N.; Palsson, B.O. iAB-RBC-283: A proteomically derived knowledge-base of erythrocyte metabolism that can be used to simulate its physiological and patho-physiological states. BMC Syst. Biol. 2011, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, R.; Nemkov, T.; D’Alessandro, A.; Grau, M.; Dietz, T.; Bohnert, B.N.; Essigke, D.; Wörn, M.; Schaefer, L.; Xiao, M.; et al. Proteinuric chronic kidney disease is associated with altered red blood cell lifespan, deformability and metabolism. Kidney Int. 2021, 100, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- La Carpia, F.; Wojczyk, B.S.; Annavajhala, M.K.; Rebbaa, A.; Culp-Hill, R.; D’Alessandro, A.; Freedberg, D.E.; Uhlemann, A.C.; Hod, E.A. Transfusional iron overload and intravenous iron infusions modify the mouse gut microbiota similarly to dietary iron. NPJ Biofilms Microbiomes 2019, 5, 26. [Google Scholar] [CrossRef]
- Hamza, I.; Dailey, H.A. One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 2012, 1823, 1617–1632. [Google Scholar] [CrossRef]
- Roy, M.K.; Cendali, F.I.; Ooyama, G.; Gamboni, F.; Morton, H.; D’Alessandro, A. Red Blood Cell Metabolism in Patients with Propionic Acidemia. Separations 2021, 8, 142. [Google Scholar] [CrossRef]
- Fraser, J.L.; Venditti, C.P. Methylmalonic and propionic acidemias: Clinical management update. Curr. Opin. Pediatr. 2016, 28, 682–693. [Google Scholar] [CrossRef]
- Nemkov, T.; Qadri, S.M.; Sheffield, W.P.; D’Alessandro, A. Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus. 2020, 18, 130–142. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Mian, S.A.; Philippe, C.; Maniati, E.; Protopapa, P.; Bergot, T.; Piganeau, M.; Nemkov, T.; Di Bella, D.; Morales, V.; Finch, A.J.; et al. Vitamin B5 and succinyl-CoA improve ineffective erythropoiesis in SF3B1-mutated myelodysplasia. Sci. Transl. Med. 2023, 15, eabn5135. [Google Scholar] [CrossRef]
- Henry, C.J.; Nemkov, T.; Casás-Selves, M.; Bilousova, G.; Zaberezhnyy, V.; Higa, K.C.; Serkova, N.J.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica 2017, 102, 1985–1994. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Sharma, R.; Sharma, M. Homocystinuria: A rare condition presenting as stroke and megaloblastic anemia. J. Pediatr. Neurosci. 2010, 5, 129–131. [Google Scholar] [CrossRef]
- Culp-Hill, R.; Zheng, C.; Reisz, J.A.; Smith, K.; Rachubinski, A.; Nemkov, T.; Butcher, E.; Granrath, R.; Hansen, K.C.; Espinosa, J.M.; et al. Red blood cell metabolism in Down syndrome: Hints on metabolic derangements in aging. Blood Adv. 2017, 1, 2776–2780. [Google Scholar] [CrossRef]
- Pogribna, M.; Melnyk, S.; Pogribny, I.; Chango, A.; Yi, P.; James, S.J. Homocysteine metabolism in children with Down syndrome: In Vitro modulation. Am. J. Hum. Genet. 2001, 69, 88–95. [Google Scholar] [CrossRef]
- Inaba, M.; Gupta, K.C.; Kuwabara, M.; Takahashi, T.; Benz, E.J., Jr.; Maede, Y. Deamidation of human erythrocyte protein 4.1: Possible role in aging. Blood 1992, 79, 3355–3361. [Google Scholar] [CrossRef]
- Janson, C.A.; Clarke, S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J. Biol. Chem. 1980, 255, 11640–11643. [Google Scholar] [CrossRef]
- Kim, E.; Lowenson, J.D.; MacLaren, D.C.; Clarke, S.; Young, S.G. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 6132–6137. [Google Scholar] [CrossRef]
- Lowenson, J.D.; Kim, E.; Young, S.G.; Clarke, S. Limited accumulation of damaged proteins in l-isoaspartyl (D-aspartyl) O-methyltransferase-deficient mice. J. Biol. Chem. 2001, 276, 20695–20702. [Google Scholar] [CrossRef]
- McFadden, P.N.; Clarke, S. Methylation at D-aspartyl residues in erythrocytes: Possible step in the repair of aged membrane proteins. Proc. Natl. Acad. Sci. USA 1982, 79, 2460–2464. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Clarke, S. Analysis of erythrocyte protein methyl esters by two-dimensional gel electrophoresis under acidic separating conditions. Anal. Biochem. 1985, 148, 79–86. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Clarke, S. Methylation of erythrocyte membrane proteins at extracellular and intracellular D-aspartyl sites in vitro. Saturation of intracellular sites in vivo. J. Biol. Chem. 1983, 258, 8485–8492. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Nemkov, T.; Dzieciatkowska, M.; Culp-Hill, R.; Stefanoni, D.; Hill, R.C.; Yoshida, T.; Dunham, A.; Kanias, T.; Dumont, L.J.; et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018, 58, 2978–2991. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Hay, A.; Dzieciatkowska, M.; Brown, B.C.; Morrison, E.J.; Hansen, K.C.; Zimring, J.C. Protein-L-isoaspartate O-methyltransferase is required for in vivo control of oxidative damage in red blood cells. Haematologica 2021, 106, 2726–2739. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.; Rosencrance, C.B.; De Vallance, E.; Giromini, A.; Williams, X.M.; Oh, J.Y.; Schmidt, H.; Straub, A.C.; Chantler, P.D.; Patel, R.P.; et al. Human and rodent red blood cells do not demonstrate xanthine oxidase activity or XO-catalyzed nitrite reduction to NO. Free Radic. Biol. Med. 2021, 174, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Georgatzakou, H.T.; Kriebardis, A.G.; Papageorgiou, E.G.; Stamoulis, K.E.; Foudoulaki-Paparizos, L.E.; Antonelou, M.H.; Papassideri, I.S. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion 2015, 55, 2659–2671. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Wu, H.; D’Alessandro, A.; Yegutkin, G.G.; Song, A.; Sun, K.; Li, J.; Cheng, N.Y.; Huang, A.; et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulation 2016, 134, 405–421. [Google Scholar] [CrossRef]
- Cakmakli, H.F.; Torres, R.J.; Menendez, A.; Yalcin-Cakmakli, G.; Porter, C.C.; Puig, J.G.; Jinnah, H.A. Macrocytic anemia in Lesch-Nyhan disease and its variants. Genet. Med. 2019, 21, 353–360. [Google Scholar] [CrossRef]
- Song, A.; Zhang, Y.; Han, L.; Yegutkin, G.G.; Liu, H.; Sun, K.; D’Alessandro, A.; Li, J.; Karmouty-Quintana, H.; Iriyama, T.; et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat. Commun. 2017, 8, 14108. [Google Scholar] [CrossRef]
- Qiang, Q.; Manalo, J.M.; Sun, H.; Zhang, Y.; Song, A.; Wen, A.Q.; Wen, Y.E.; Chen, C.; Liu, H.; Cui, Y.; et al. Erythrocyte adenosine A2B receptor prevents cognitive and auditory dysfunction by promoting hypoxic and metabolic reprogramming. PLoS Biol. 2021, 19, e3001239. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef]
- Sun, K.; D’Alessandro, A.; Ahmed, M.H.; Zhang, Y.; Song, A.; Ko, T.P.; Nemkov, T.; Reisz, J.A.; Wu, H.; Adebiyi, M.; et al. Structural and Functional Insight of Sphingosine 1-Phosphate-Mediated Pathogenic Metabolic Reprogramming in Sickle Cell Disease. Sci. Rep. 2017, 7, 15281. [Google Scholar] [CrossRef]
- Hay, A.; Nemkov, T.; Gamboni, F.; Dzieciatkowska, M.; Key, A.; Galbraith, M.; Bartsch, K.; Sun, K.; Xia, Y.; Stone, M.; et al. Sphingosine 1-phosphate has a negative effect on RBC storage quality. Blood Adv. 2023, 7, 1379–1393. [Google Scholar] [CrossRef]
- Thomas, T.; Cendali, F.; Fu, X.; Gamboni, F.; Morrison, E.J.; Beirne, J.; Nemkov, T.; Antonelou, M.H.; Kriebardis, A.; Welsby, I.; et al. Fatty acid desaturase activity in mature red blood cells and implications for blood storage quality. Transfusion 2021, 61, 1867–1883. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Q.; Kao, Y.R.; Diaz, A.; Tasset, I.; Kaushik, S.; Thiruthuvanathan, V.; Zintiridou, A.; Nieves, E.; Dzieciatkowska, M.; et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature 2021, 591, 117–123. [Google Scholar] [CrossRef]
- Kim, C.Y.; Johnson, H.; Peltier, S.; Spitalnik, S.L.; Hod, E.A.; Francis, R.O.; Hudson, K.E.; Stone, E.F.; Gordy, D.E.; Fu, X.; et al. Deuterated Linoleic Acid Attenuates the RBC Storage Lesion in a Mouse Model of Poor RBC Storage. Front. Physiol. 2022, 13, 868578. [Google Scholar] [CrossRef]
- Howie, H.L.; Hay, A.M.; de Wolski, K.; Waterman, H.; Lebedev, J.; Fu, X.; Culp-Hill, R.; D’Alessandro, A.; Gorham, J.D.; Ranson, M.S.; et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019, 3, 2272–2285. [Google Scholar] [CrossRef]
- Fisher, A.B. The phospholipase A(2) activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef]
- Nemkov, T.; Skinner, S.C.; Nader, E.; Stefanoni, D.; Robert, M.; Cendali, F.; Stauffer, E.; Cibiel, A.; Boisson, C.; Connes, P.; et al. Acute Cycling Exercise Induces Changes in Red Blood Cell Deformability and Membrane Lipid Remodeling. Int. J. Mol. Sci. 2021, 22, 896. [Google Scholar] [CrossRef]
- Nemkov, T.; Skinner, S.; Diaw, M.; Diop, S.; Samb, A.; Connes, P.; D’Alessandro, A. Plasma Levels of Acyl-Carnitines and Carboxylic Acids Correlate With Cardiovascular and Kidney Function in Subjects With Sickle Cell Trait. Front. Physiol. 2022, 13, 916197. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nouraie, S.M.; Zhang, Y.; Cendali, F.; Gamboni, F.; Reisz, J.A.; Zhang, X.; Bartsch, K.W.; Galbraith, M.D.; Gordeuk, V.R.; et al. In Vivo evaluation of the effect of sickle cell hemoglobin S, C and therapeutic transfusion on erythrocyte metabolism and cardiorenal dysfunction. Am. J. Hematol. 2023, 98, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Bogdanov, M.; Zhang, Y.; Sun, K.; Zhao, S.; Song, A.; Luo, R.; Parchim, N.F.; Liu, H.; Huang, A.; et al. Hypoxia-mediated impaired erythrocyte Lands’ Cycle is pathogenic for sickle cell disease. Sci. Rep. 2016, 6, 29637. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Reisz, J.A.; Zhang, Y.; Gehrke, S.; Alexander, K.; Kanias, T.; Triulzi, D.J.; Donadee, C.; Barge, S.; Badlam, J.; et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019, 3, 884–896. [Google Scholar] [CrossRef]
- Kumar, S.; Vassallo, J.D.; Nattamai, K.J.; Hassan, A.; Karns, R.; Vollmer, A.; Soller, K.; Sakk, V.; Sacma, M.; Nemkov, T.; et al. pH regulates hematopoietic stem cell potential via polyamines. EMBO Rep. 2023, 24, e55373. [Google Scholar] [CrossRef] [PubMed]
- Kalani Roy, M.; La Carpia, F.; Cendali, F.; Fernando, S.; Moriconi, C.; Wojczyk, B.S.; Wang, L.; Nemkov, T.; Hod, E.A.; D’Alessandro, A. Irradiation Causes Alterations of Polyamine, Purine, and Sulfur Metabolism in Red Blood Cells and Multiple Organs. J. Proteome Res. 2022, 21, 519–534. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozüyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Detterich, J.A.; Connes, P. Nitric oxide, vasodilation and the red blood cell. Biorheology 2014, 51, 121–134. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Wang, B.; Liu, H.; Shah, H.; Carmona-Fontaine, C.; Rustenburg, A.S.; Salah, S.; Gunner, M.R.; Chodera, J.D.; Cross, J.R.; et al. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat. Chem. Biol. 2017, 13, 494–500. [Google Scholar] [CrossRef]
- Roy, M.K.; Wilkerson, R.B.; Alexander, K.; Nokoff, N.J.; Cree-Green, M.; D’Alessandro, A. Longitudinal metabolic study of red blood cells from patients undergoing gender-affirming testosterone therapy. Blood Adv. 2022. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Yoshida, T.; Bordbar, A.; Palsson, B.O.; Hansen, K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017, 57, 325–336. [Google Scholar] [CrossRef]
- Bordbar, A.; Yurkovich, J.T.; Paglia, G.; Rolfsson, O.; Sigurjónsson, Ó.E.; Palsson, B.O. Elucidating dynamic metabolic physiology through network integration of quantitative time-course metabolomics. Sci. Rep. 2017, 7, 46249. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Yoshida, T.; Dunham, A.; Wen, E.Y.; Wen, A.Q.; Roach, R.C.; Hansen, K.C.; Xia, Y.; et al. Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage. Front. Med. 2017, 4, 175. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Howie, H.L.; Hay, A.M.; Dziewulska, K.H.; Brown, B.C.; Wither, M.J.; Karafin, M.; Stone, E.F.; Spitalnik, S.L.; Hod, E.A.; et al. Hematologic and systemic metabolic alterations due to Mediterranean class II G6PD deficiency in mice. JCI Insight 2021, 6, e147056. [Google Scholar] [CrossRef]
- Moriconi, C.; Dzieciatkowska, M.; Roy, M.; D’Alessandro, A.; Roingeard, P.; Lee, J.Y.; Gibb, D.R.; Tredicine, M.; McGill, M.A.; Qiu, A.; et al. Retention of functional mitochondria in mature red blood cells from patients with sickle cell disease. Br. J. Haematol. 2022, 198, 574–586. [Google Scholar] [CrossRef]
- Jagadeeswaran, R.; Vazquez, B.A.; Thiruppathi, M.; Ganesh, B.B.; Ibanez, V.; Cui, S.; Engel, J.D.; Diamond, A.M.; Molokie, R.E.; DeSimone, J.; et al. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp. Hematol. 2017, 50, 46–52. [Google Scholar] [CrossRef]
- Esperti, S.; Nader, E.; Stier, A.; Boisson, C.; Carin, R.; Marano, M.; Robert, M.; Martin, M.; Horand, F.; Cibiel, A.; et al. Increased retention of functional mitochondria in mature sickle red blood cells is associated with increased sickling tendency, hemolysis and oxidative stress. Haematologica 2023. [Google Scholar] [CrossRef]
- Tumburu, L.; Ghosh-Choudhary, S.; Seifuddin, F.T.; Barbu, E.A.; Yang, S.; Ahmad, M.M.; Wilkins, L.H.W.; Tunc, I.; Sivakumar, I.; Nichols, J.S.; et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood 2021, 137, 3116–3126. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Caielli, S.; Cardenas, J.; de Jesus, A.A.; Baisch, J.; Walters, L.; Blanck, J.P.; Balasubramanian, P.; Stagnar, C.; Ohouo, M.; Hong, S.; et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 2021, 184, 4464–4479.e4419. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Tundo, G.R.; Campagnolo, L.; Valacchi, G.; Orlandi, A.; Curatolo, P.; Borsellino, G.; D’Esposito, M.; Ciaccio, C.; Cesare, S.D.; et al. Retention of Mitochondria in Mature Human Red Blood Cells as the Result of Autophagy Impairment in Rett Syndrome. Sci. Rep. 2017, 7, 12297. [Google Scholar] [CrossRef]
- Thomas, T.A.; Qiu, A.; Kim, C.Y.; Gordy, D.E.; Miller, A.; Tredicine, M.; Dzieciatkowska, M.; Zotti, F.D.; Hod, E.A.; D’Alessandro, A.; et al. Reticulocytes in donor RBC units enhance RBC alloimmunization. bioRxiv 2023. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.K.; Culp-Hill, R.; Ludwig, M.P.; Smith, K.P.; Waugh, K.A.; Minter, R.; Tuttle, K.D.; Lewis, H.C.; Rachubinski, A.L.; Granrath, R.E.; et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nat. Commun. 2019, 10, 4766. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015, 55, 205–219. [Google Scholar] [CrossRef]
- LaCroix, I.S.; Cohen, M.; Moore, E.E.; Dzieciatkowska, M.; Nemkov, T.; Schaid, T.R., Jr.; Debot, M.; Jones, K.; Silliman, C.C.; Hansen, K.C.; et al. Omics Markers of Red Blood Cell Transfusion in Trauma. Int. J. Mol. Sci. 2022, 23, 13815. [Google Scholar] [CrossRef]
- Culp-Hill, R.; Srinivasan, A.J.; Gehrke, S.; Kamyszek, R.; Ansari, A.; Shah, N.; Welsby, I.; D’Alessandro, A. Effects of red blood cell (RBC) transfusion on sickle cell disease recipient plasma and RBC metabolism. Transfusion 2018, 58, 2797–2806. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Hess, J.R.; D’Alessandro, A. Red blood cell metabolism and preservation. In Rossi’s Principles of Transfusion Medicine; Wiley: Hoboken, NJ, USA, 2022; pp. 143–157. [Google Scholar]
- D’Alessandro, A.; D’Amici, G.M.; Vaglio, S.; Zolla, L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica 2012, 97, 107–115. [Google Scholar] [CrossRef]
- Yurkovich, J.T.; Zielinski, D.C.; Yang, L.; Paglia, G.; Rolfsson, O.; Sigurjónsson, Ó.E.; Broddrick, J.T.; Bordbar, A.; Wichuk, K.; Brynjólfsson, S.; et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J. Biol. Chem. 2017, 292, 19556–19564. [Google Scholar] [CrossRef]
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef]
- D’Alessandro, A. Red Blood Cell Omics and Machine Learning in Transfusion Medicine: Singularity Is Near. Transfus. Med. Hemotherapy 2023, 1–10. [Google Scholar] [CrossRef]
- Gevi, F.; D’Alessandro, A.; Rinalducci, S.; Zolla, L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J. Proteom. 2012, 76, 168–180. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Korsten, H.; van Bruggen, R.; de Korte, D. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion 2018, 58, 1992–2002. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Hansen, K.C.; Szczepiorkowski, Z.M.; Dumont, L.J. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: A metabolomics approach. Transfusion 2015, 55, 2955–2966. [Google Scholar] [CrossRef]
- Yoshida, T.; Blair, A.; D’Alessandro, A.; Nemkov, T.; Dioguardi, M.; Silliman, C.C.; Dunham, A. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus. 2017, 15, 172–181. [Google Scholar] [CrossRef]
- Kriebardis, A.G.; Antonelou, M.H.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J. Cell. Mol. Med. 2007, 11, 148–155. [Google Scholar] [CrossRef]
- Rinalducci, S.; Ferru, E.; Blasi, B.; Turrini, F.; Zolla, L. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012, 10 (Suppl. S2), s55–s62. [Google Scholar] [CrossRef]
- Rogers, S.C.; Ross, J.G.; d’Avignon, A.; Gibbons, L.B.; Gazit, V.; Hassan, M.N.; McLaughlin, D.; Griffin, S.; Neumayr, T.; Debaun, M.; et al. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood 2013, 121, 1651–1662. [Google Scholar] [CrossRef]
- de Bruin, S.; Peters, A.L.; Wijnberge, M.; van Baarle, F.; AbdelRahman, A.H.A.; Vermeulen, C.; Beuger, B.M.; Reisz, J.A.; D’Alessandro, A.; Vlaar, A.P.J.; et al. Storage of red blood cells in alkaline PAGGGM improves metabolism but has no effect on recovery after transfusion. Blood Adv. 2022, 6, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Shin, H.K.H.; Baek, J.H.; Champagne, D.P.; Nemkov, T.; Thomas, T.; Francis, R.O.; Zimring, J.C.; Yoshida, T.; Reisz, J.A.; et al. Red blood cell metabolism in Rhesus macaques and humans: Comparative biology of blood storage. Haematologica 2020, 105, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Bertolone, L.; Shin, H.K.; Stefanoni, D.; Baek, J.H.; Gao, Y.; Morrison, E.J.; Nemkov, T.; Thomas, T.; Francis, R.O.; Hod, E.A.; et al. ZOOMICS: Comparative Metabolomics of Red Blood Cells from Old World Monkeys and Humans. Front. Physiol. 2020, 11, 593841. [Google Scholar] [CrossRef] [PubMed]
- Zimring, J.C.; Smith, N.; Stowell, S.R.; Johnsen, J.M.; Bell, L.N.; Francis, R.O.; Hod, E.A.; Hendrickson, J.E.; Roback, J.D.; Spitalnik, S.L. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion 2014, 54, 137–148. [Google Scholar] [CrossRef]
- Williams, A.T.; Jani, V.P.; Nemkov, T.; Lucas, A.; Yoshida, T.; Dunham, A.; D’Alessandro, A.; Cabrales, P. Transfusion of Anaerobically or Conventionally Stored Blood After Hemorrhagic Shock. Shock 2020, 53, 352–362. [Google Scholar] [CrossRef]
- Bertolone, L.; Shin, H.K.H.; Baek, J.H.; Gao, Y.; Spitalnik, S.L.; Buehler, P.W.; D’Alessandro, A. ZOOMICS: Comparative Metabolomics of Red Blood Cells From Guinea Pigs, Humans, and Non-human Primates During Refrigerated Storage for Up to 42 Days. Front. Physiol. 2022, 13, 845347. [Google Scholar] [CrossRef]
- Miglio, A.; Maslanka, M.; Di Tommaso, M.; Rocconi, F.; Nemkov, T.; Buehler, P.W.; Antognoni, M.T.; Spitalnik, S.L.; D’Alessandro, A. ZOOMICS: Comparative metabolomics of red blood cells from dogs, cows, horses and donkeys during refrigerated storage for up to 42 days. Blood Transfus. 2022. [Google Scholar] [CrossRef]
- Miglio, A.; Cremonini, V.; Leonardi, L.; Manuali, E.; Coliolo, P.; Barbato, O.; Dall’Aglioa, C.; Antognoni, M.T. Omics Technologies in Veterinary Medicine: Literature Review and Perspectives in Transfusion Medicine. Transfus. Med. Hemotherapy 2023, 5, 198–207. [Google Scholar] [CrossRef]
- Mykhailova, O.; Olafson, C.; Turner, T.R.; D’Alessandro, A.; Acker, J.P. Donor-dependent aging of young and old red blood cell subpopulations: Metabolic and functional heterogeneity. Transfusion 2020, 60, 2633–2646. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Tzounakas, V.L.; Dzieciatkowska, M.; Arvaniti, V.-Z.; Papageorgiou, E.G.; Papassideri, I.S.; Stamoulis, K.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Innate Variability in Physiological and Omics Aspects of the Beta Thalassemia Trait-Specific Donor Variation Effects. Front. Physiol. 2022, 13, 999. [Google Scholar] [CrossRef]
- Raval, J.S.; Waters, J.H.; Seltsam, A.; Scharberg, E.A.; Richter, E.; Kameneva, M.V.; Yazer, M.H. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011, 100, 418–421. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Drossos, P.V.; Karadimas, D.G.; Valsami, S.; Stamoulis, K.E.; Papassideri, I.S.; Politou, M.; Antonelou, M.H.; Kriebardis, A.G. Sex-related aspects of the red blood cell storage lesion. Blood Transfus. 2021, 19, 224–236. [Google Scholar] [CrossRef]
- Fu, X.; Felcyn, J.R.; Odem-Davis, K.; Zimring, J.C. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: A targeted metabolomics study. Transfusion 2016, 56, 2560–2570. [Google Scholar] [CrossRef]
- D’Alessandro, A. In vivo clearance of stored red blood cells. Blood 2021, 137, 2275–2276. [Google Scholar] [CrossRef]
- Roussel, C.; Morel, A.; Dussiot, M.; Marin, M.; Colard, M.; Fricot-Monsinjon, A.; Martinez, A.; Chambrion, C.; Henry, B.; Casimir, M.; et al. Rapid clearance of storage-induced microerythrocytes alters transfusion recovery. Blood 2021, 137, 2285–2298. [Google Scholar] [CrossRef]
- Kerkelä, E.; Lahtela, J.; Larjo, A.; Impola, U.; Mäenpää, L.; Mattila, P. Exploring Transcriptomic Landscapes in Red Blood Cells, in Their Extracellular Vesicles and on a Single-Cell Level. Int. J. Mol. Sci. 2022, 23, 12897. [Google Scholar] [CrossRef]
- Nemkov, T.; Yoshida, T.; Nikulina, M.; D’Alessandro, A. High-Throughput Metabolomics Platform for the Rapid Data-Driven Development of Novel Additive Solutions for Blood Storage. Front. Physiol. 2022, 13, 833242. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Kelher, M.; West, F.B.; Schwindt, R.K.; Banerjee, A.; Moore, E.E.; Silliman, C.C.; Hansen, K.C. Routine storage of red blood cell (RBC) units in additive solution-3: A comprehensive investigation of the RBC metabolome. Transfusion 2015, 55, 1155–1168. [Google Scholar] [CrossRef]
- Rolfsson, Ó.; Sigurjonsson, Ó.E.; Magnusdottir, M.; Johannsson, F.; Paglia, G.; Guðmundsson, S.; Bordbar, A.; Palsson, S.; Brynjólfsson, S.; Guðmundsson, S.; et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017, 112, 326–335. [Google Scholar] [CrossRef]
- Bordbar, A.; Johansson, P.I.; Paglia, G.; Harrison, S.J.; Wichuk, K.; Magnusdottir, M.; Valgeirsdottir, S.; Gybel-Brask, M.; Ostrowski, S.R.; Palsson, S.; et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion 2016, 56, 852–862. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Hansen, K.C.; Silliman, C.C.; Moore, E.E.; Kelher, M.; Banerjee, A. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015, 108, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yurkovich, J.T.; Yang, L.; Palsson, B.O. Biomarkers are used to predict quantitative metabolite concentration profiles in human red blood cells. PLoS Comput. Biol. 2017, 13, e1005424. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Thomas, T.; Akpan, I.J.; Reisz, J.A.; Cendali, F.I.; Gamboni, F.; Nemkov, T.; Thangaraju, K.; Katneni, U.; Tanaka, K.; et al. Biological and Clinical Factors Contributing to the Metabolic Heterogeneity of Hospitalized Patients with and without COVID-19. Cells 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Dumont, L.J.; D’Alessandro, A.; Szczepiorkowski, Z.M.; Yoshida, T. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion 2016, 56, 392–403. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Gevi, F.; Zolla, L. Red blood cell metabolism under prolonged anaerobic storage. Mol. Biosyst. 2013, 9, 1196–1209. [Google Scholar] [CrossRef]
- Rabcuka, J.; Blonski, S.; Meli, A.; Sowemimo-Coker, S.; Zaremba, D.; Stephenson, D.; Dzieciatkowska, M.; Nerguizian, D.; Cardigan, R.; Korczyk, P.M.; et al. Metabolic reprogramming under hypoxic storage preserves faster oxygen unloading from stored red blood cells. Blood Adv. 2022, 6, 5415–5428. [Google Scholar] [CrossRef]
- Hay, A.; Dziewulska, K.; Gamboni, F.; Nerguizian, D.; Dzieciatkowska, M.; Zimring, J.C.; D’Alessandro, A. Hypoxic storage of murine red blood cells improves energy metabolism and post-transfusion recoveries. Blood Transfus. 2022, 21. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Yoshida, T.; Nestheide, S.; Nemkov, T.; Stocker, S.; Stefanoni, D.; Mohmoud, F.; Rugg, N.; Dunham, A.; Cancelas, J.A. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion 2020, 60, 786–798. [Google Scholar] [CrossRef]
- Dumont, L.J.; AuBuchon, J.P. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 2008, 48, 1053–1060. [Google Scholar] [CrossRef]
- Roubinian, N.H.; Reese, S.E.; Qiao, H.; Plimier, C.; Fang, F.; Page, G.P.; Cable, R.G.; Custer, B.; Gladwin, M.T.; Goel, R.; et al. Donor genetic and nongenetic factors affecting red blood cell transfusion effectiveness. JCI Insight 2022, 7, e152598. [Google Scholar] [CrossRef]
- Hazegh, K.; Fang, F.; Bravo, M.D.; Tran, J.Q.; Muench, M.O.; Jackman, R.P.; Roubinian, N.; Bertolone, L.; D’Alessandro, A.; Dumont, L.; et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion 2021, 61, 435–448. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Paronis, E.C.; Arvaniti, V.Z.; Velentzas, A.D.; Apostolidou, A.C.; Balafas, E.G.; Dzieciatkowska, M.; Kostomitsopoulos, N.G.; Stamoulis, K.; Papassideri, I.S.; et al. The Post-Storage Performance of RBCs from Beta-Thalassemia Trait Donors Is Related to Their Storability Profile. Int. J. Mol. Sci. 2021, 22, 12281. [Google Scholar] [CrossRef]
- van ‘t Erve, T.J.; Doskey, C.M.; Wagner, B.A.; Hess, J.R.; Darbro, B.W.; Ryckman, K.K.; Murray, J.C.; Raife, T.J.; Buettner, G.R. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic. Biol. Med. 2014, 76, 107–113. [Google Scholar] [CrossRef]
- Van ‘t Erve, T.J.; Wagner, B.A.; Martin, S.M.; Knudson, C.M.; Blendowski, R.; Keaton, M.; Holt, T.; Hess, J.R.; Buettner, G.R.; Ryckman, K.K.; et al. The heritability of hemolysis in stored human red blood cells. Transfusion 2015, 55, 1178–1185. [Google Scholar] [CrossRef]
- van ‘t Erve, T.J.; Wagner, B.A.; Martin, S.M.; Knudson, C.M.; Blendowski, R.; Keaton, M.; Holt, T.; Hess, J.R.; Buettner, G.R.; Ryckman, K.K.; et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion 2014, 54, 2055–2063. [Google Scholar] [CrossRef]
- Hay, A.M.; Howie, H.L.; Gorham, J.D.; D’Alessandro, A.; Spitalnik, S.L.; Hudson, K.E.; Zimring, J.C. Mouse background genetics in biomedical research: The devil’s in the details. Transfusion 2021, 61, 3017–3025. [Google Scholar] [CrossRef]
- Endres-Dighe, S.M.; Guo, Y.; Kanias, T.; Lanteri, M.; Stone, M.; Spencer, B.; Cable, R.G.; Kiss, J.E.; Kleinman, S.; Gladwin, M.T.; et al. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion 2019, 59, 46–56. [Google Scholar] [CrossRef]
- Josephson, C.D.; Glynn, S.; Mathew, S.; Birch, R.; Bakkour, S.; Baumann Kreuziger, L.; Busch, M.P.; Chapman, K.; Dinardo, C.; Hendrickson, J.; et al. The Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P): A research program striving to improve blood donor safety and optimize transfusion outcomes across the lifespan. Transfusion 2022, 62, 982–999. [Google Scholar] [CrossRef]
- Lanteri, M.C.; Kanias, T.; Keating, S.; Stone, M.; Guo, Y.; Page, G.P.; Brambilla, D.J.; Endres-Dighe, S.M.; Mast, A.E.; Bialkowski, W.; et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: Results of recall phase of the REDS-III RBC-Omics study. Transfusion 2019, 59, 79–88. [Google Scholar] [CrossRef]
- Guo, Y.; Busch, M.P.; Seielstad, M.; Endres-Dighe, S.; Westhoff, C.M.; Keating, B.; Hoppe, C.; Bordbar, A.; Custer, B.; Butterworth, A.S.; et al. Development and evaluation of a transfusion medicine genome wide genotyping array. Transfusion 2019, 59, 101–111. [Google Scholar] [CrossRef]
- Roubinian, N.H.; Plimier, C.; Woo, J.P.; Lee, C.; Bruhn, R.; Liu, V.X.; Escobar, G.J.; Kleinman, S.H.; Triulzi, D.J.; Murphy, E.L.; et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood 2019, 134, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Busch, M.P.; Dziewulska, K.; Francis, R.O.; Hod, E.A.; Zimring, J.C.; D’Alessandro, A.; Page, G.P. Genome-wide metabolite quantitative trait loci analysis (mQTL) in red blood cells from volunteer blood donors. J. Biol. Chem. 2022, 298, 102706. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Stefanoni, D.; Bordbar, A.; Issaian, A.; Palsson, B.O.; Dumont, L.J.; Hay, A.M.; Song, A.; Xia, Y.; Redzic, J.S.; et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight 2021, 6, e146175. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Fu, X.; Reisz, J.A.; Kanias, T.; Nemkov, T.; Page, G.P.; Dumont, L.; Roubinian, N.; Stone, M.; Kleinman, S.; et al. Nicotine exposure increases markers of oxidant stress in stored red blood cells from healthy donor volunteers. Transfusion 2020, 60, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, R.A.; Plimier, C.; Lee, C.; Kanias, T.; Cushing, M.M.; Sachais, B.S.; Kleinman, S.; Busch, M.P.; Roubinian, N.H. Additive effects of blood donor smoking and gamma irradiation on outcome measures of red blood cell transfusion. Transfusion 2020, 60, 1175–1182. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Fu, X.; Reisz, J.A.; Stone, M.; Kleinman, S.; Zimring, J.C.; Busch, M. Ethyl glucuronide, a marker of alcohol consumption, correlates with metabolic markers of oxidant stress but not with hemolysis in stored red blood cells from healthy blood donors. Transfusion 2020, 60, 1183–1196. [Google Scholar] [CrossRef]
- Bertolone, L.; Roy, M.K.; Hay, A.M.; Morrison, E.J.; Stefanoni, D.; Fu, X.; Kanias, T.; Kleinman, S.; Dumont, L.J.; Stone, M.; et al. Impact of taurine on red blood cell metabolism and implications for blood storage. Transfusion 2020, 60, 1212–1226. [Google Scholar] [CrossRef]
- Hill, H.R.; Oliver, C.K.; Lippert, L.E.; Greenwalt, T.J.; Hess, J.R. The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sang. 2001, 81, 161–166. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Hansen, K.C. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. 2016, 14, 140–144. [Google Scholar] [CrossRef]
- Swift, L.M.; Roberts, A.; Pressman, J.; Guerrelli, D.; Allen, S.; Haq, K.T.; Reisz, J.A.; D’Alessandro, A.; Posnack, N.G. Toxins in plastic: Evidence for the cardiodepressive effects of di-2-ethylhexylphthalate (DEHP). bioRxiv 2023. [Google Scholar] [CrossRef]
- Gasiorowski, R.; Forbes, M.K.; Silver, G.; Krastev, Y.; Hamdorf, B.; Lewis, B.; Tisbury, M.; Cole-Sinclair, M.; Lanphear, B.P.; Klein, R.A.; et al. Effect of Plasma and Blood Donations on Levels of Perfluoroalkyl and Polyfluoroalkyl Substances in Firefighters in Australia: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e226257. [Google Scholar] [CrossRef]
- Himbert, S.; D’Alessandro, A.; Qadri, S.M.; Majcher, M.J.; Hoare, T.; Sheffield, W.P.; Nagao, M.; Nagle, J.F.; Rheinstädter, M.C. The bending rigidity of the red blood cell cytoplasmic membrane. PLoS ONE 2022, 17, e0269619. [Google Scholar] [CrossRef]
- Himbert, S.; Qadri, S.M.; Sheffield, W.P.; Schubert, P.; D’Alessandro, A.; Rheinstädter, M.C. Blood bank storage of red blood cells increases RBC cytoplasmic membrane order and bending rigidity. PLoS ONE 2021, 16, e0259267. [Google Scholar] [CrossRef]
- Bicalho, B.; Holovati, J.L.; Acker, J.P. Phospholipidomics reveals differences in glycerophosphoserine profiles of hypothermically stored red blood cells and microvesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 317–326. [Google Scholar] [CrossRef]
- Huyut, Z.; Şekeroğlu, M.R.; Balahoroğlu, R.; Huyut, M.T. Characteristics of resveratrol and serotonin on antioxidant capacity and susceptibility to oxidation of red blood cells in stored human blood in a time-dependent manner. J. Int. Med. Res. 2018, 46, 272–283. [Google Scholar] [CrossRef]
- Antosik, A.; Czubak, K.; Cichon, N.; Nowak, P.; Zbikowska, H. Vitamin E Analogue Protects Red Blood Cells against Storage-Induced Oxidative Damage. Transfus. Med. Hemother. 2018, 45, 347–354. [Google Scholar] [CrossRef]
- Pallotta, V.; Gevi, F.; D’Alessandro, A.; Zolla, L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: A metabolomics overview. Blood Transfus. 2014, 12, 376–387. [Google Scholar] [CrossRef]
- Stowell, S.R.; Smith, N.H.; Zimring, J.C.; Fu, X.; Palmer, A.F.; Fontes, J.; Banerjee, U.; Yazer, M.H. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion 2013, 53, 2248–2257. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Arduini, A. L-carnitine and its possible role in red cell and platelet storage. Transfus. Med. Rev. 2004, 18, 58–65. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Açaì (Euterpe oleracea) Extract Protects Human Erythrocytes from Age-Related Oxidative Stress. Cells 2022, 11, 2391. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant Activity of Quercetin in a H2O2-Induced Oxidative Stress Model in Red Blood Cells: Functional Role of Band 3 Protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence for structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Catala, A.; Stone, M.; Busch, M.P.; D’Alessandro, A. Reprogramming of red blood cell metabolism in Zika virus–infected donors. Transfusion 2022, 62, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The Effect of Sepsis on the Erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Zimring, J.C.; Busch, M. Chronological storage age and metabolic age of stored red blood cells: Are they the same? Transfusion 2019, 59, 1620–1623. [Google Scholar] [CrossRef]
- Marrocco, C.; Pallotta, V.; D’Alessandro, A.; Alves, G.; Zolla, L. Red blood cell populations and membrane levels of peroxiredoxin 2 as candidate biomarkers to reveal blood doping. Blood Transfus. 2012, 10 (Suppl. S2), s71–s77. [Google Scholar] [CrossRef]
- Scott, A.V.; Nagababu, E.; Johnson, D.J.; Kebaish, K.M.; Lipsitz, J.A.; Dwyer, I.M.; Zuckerberg, G.S.; Barodka, V.M.; Berkowitz, D.E.; Frank, S.M. 2,3-Diphosphoglycerate Concentrations in Autologous Salvaged Versus Stored Red Blood Cells and in Surgical Patients After Transfusion. Anesth. Analg. 2016, 122, 616–623. [Google Scholar] [CrossRef]
- Hay, A.; Howie, H.L.; Waterman, H.R.; de Wolski, K.; Zimring, J.C. Murine red blood cells from genetically distinct donors cross-regulate when stored together. Transfusion 2017, 57, 2657–2664. [Google Scholar] [CrossRef]
- Figueroa, D. The Diego blood group system: A review. Immunohematology 2013, 29, 73–81. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, A.; Anastasiadi, A.T.; Tzounakas, V.L.; Nemkov, T.; Reisz, J.A.; Kriebardis, A.G.; Zimring, J.C.; Spitalnik, S.L.; Busch, M.P. Red Blood Cell Metabolism In Vivo and In Vitro. Metabolites 2023, 13, 793. https://doi.org/10.3390/metabo13070793
D’Alessandro A, Anastasiadi AT, Tzounakas VL, Nemkov T, Reisz JA, Kriebardis AG, Zimring JC, Spitalnik SL, Busch MP. Red Blood Cell Metabolism In Vivo and In Vitro. Metabolites. 2023; 13(7):793. https://doi.org/10.3390/metabo13070793
Chicago/Turabian StyleD’Alessandro, Angelo, Alkmini T. Anastasiadi, Vassilis L. Tzounakas, Travis Nemkov, Julie A. Reisz, Anastsios G. Kriebardis, James C. Zimring, Steven L. Spitalnik, and Michael P. Busch. 2023. "Red Blood Cell Metabolism In Vivo and In Vitro" Metabolites 13, no. 7: 793. https://doi.org/10.3390/metabo13070793