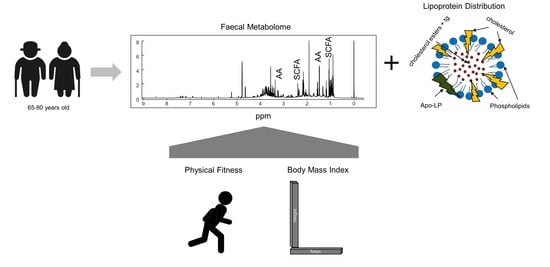
Human Fecal Metabolome Reflects Differences in Body Mass Index, Physical Fitness, and Blood Lipoproteins in Healthy Older Adults
Abstract
:1. Introduction
2. Results
2.1. Human Fecal Metabolome of Older Adults
2.2. Associations between the Human Fecal Metabolome and Physical Fitness
2.3. Fecal Metabolic Differences between Overweight/Obese and Normal BMI Older Adults
2.4. Associations between Blood Lipoproteins and the Fecal Metabolome
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Chemicals
4.3. Sample Preparation for Metabolomics Analysis
4.3.1. Fecal Sample Preparation for 1H NMR Metabolomics Analysis
4.3.2. Fecal Sample Preparation for GC-MS Metabolomics Analysis
4.4. Data Acquisition
4.4.1. 1H NMR Spectroscopy Analysis
4.4.2. GC-MS Data Acquisition
4.5. Processing of the Raw 1H NMR Spectra and GC-MS Data
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karu, N.; Deng, L.; Slae, M.; Guo, A.C.; Sajed, T.; Huynh, H.; Wine, E.; Wishart, D.S. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. Anal. Chim. Acta 2018, 1030, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ganobis, C.M.; Al-Abdul-Wahid, M.S.; Renwick, S.; Yen, S.; Carriero, C.; Aucoin, M.G.; Allen-Vercoe, E. 1D 1 H NMR as a Tool for Fecal Metabolomics. Curr. Protoc. Chem. Biol. 2020, 12, e83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lorenzen, J.K.; Astrup, A.; Larsen, L.H.; Yde, C.C.; Clausen, M.R.; Bertram, H.C. Metabolic Effects of a 24-Week Energy-Restricted Intervention Combined with Low or High Dairy Intake in Overweight Women: An NMR-Based Metabolomics Investigation. Nutrients 2016, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, F.-P.J.; Sprenger, N.; Montoliu, I.; Rezzi, S.; Kochhar, S.; Nicholson, J. Dietary Modulation of Gut Functional Ecology Studied by Fecal Metabonomics. J. Proteome Res. 2010, 9, 5284–5295. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Rigottier-Gois, L.; Lay, C.; Lepage, P.; Podglajen, I.; Marteau, P.; Doré, J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 106–111. [Google Scholar] [CrossRef]
- Palomino-Schätzlein, M.; Mayneris-Perxachs, J.; Caballano-Infantes, E.; Rodríguez, M.A.; Palomo-Buitrago, M.-E.; Xiao, X.; Mares, R.; Ricart, W.; Simó, R.; Herance, J.R.; et al. Combining metabolic profiling of plasma and faeces as a fingerprint of insulin resistance in obesity. Clin. Nutr. 2020, 39, 2292–2300. [Google Scholar] [CrossRef]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Ransom, T.P. Dietary fiber, the evolution of the human diet and coronary heart disease. Nutr. Res. 1998, 18, 633–652. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Rademaker, M.; Cutfield, W.S.; Pinto, T.E.; Tregurtha, S.; Faherty, A.; Peart, J.M.; Drury, P.L.; Hofman, P.L. Effects of Age, Gender, BMI, and Anatomical Site on Skin Thickness in Children and Adults with Diabetes. PLoS ONE 2014, 9, e86637. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. N. Eng. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Castro-Mejía, J.L.; Khakimov, B.; Krych, Ł.; Bülow, J.; Bechshøft, R.L.; Højfeldt, G.; Mertz, K.H.; Garne, E.S.; Schacht, S.R.; Ahmad, H.F.; et al. Physical fitness in community-dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell 2020, 19, e13105. [Google Scholar] [CrossRef] [Green Version]
- Moludi, J.; Maleki, V.; Jafari-Vayghan, H.; Vaghef-Mehrabany, E.; Alizadeh, M. Metabolic endotoxemia and cardiovascular disease: A systematic review about potential roles of prebiotics and probiotics. Clin. Exp. Pharmacol. Physiol. 2020, 47, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Trimigno, A.; Aru, V.; Khakimov, B.; Engelsen, S.B. Human Faecal 1H NMR Metabolomics: Evaluation of Solvent and Sample Processing on Coverage and Reproducibility of Signature Metabolites. Anal. Chem. 2020, 92, 9546–9555. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Christiansen, L.D.; Heins, A.; Sørensen, K.M.; Schöller, C.E.G.; Clausen, A.; Skov, T.; Gernaey, K.V.; Engelsen, S.B. Untargeted GC-MS Metabolomics Reveals Changes in the Metabolite Dynamics of Industrial Scale Batch Fermentations of Streptoccoccus thermophilus Broth. Biotechnol. J. 2017, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Aru, V.; Lam, C.; Khakimov, B.; Hoefsloot, H.C.; Zwanenburg, G.; Lind, M.V.; Schäfer, H.; van Duynhoven, J.; Jacobs, D.M.; Smilde, A.K.; et al. Quantification of lipoprotein profiles by nuclear magnetic resonance spectroscopy and multivariate data analysis. TrAC Trends Anal. Chem. 2017, 94, 210–219. [Google Scholar] [CrossRef]
- Khakimov, B.; Huub, C.J.H.; Mobaraki, N.; Violetta, A.; Kristensen, M.; Mads, V.L.; Holm, L.; Josué, L.C.; Dennis, S.N.; Doris, M.J.; et al. Human blood lipoprotein predictions from 1H NMR spectra: Protocol, model performances and cage of covariance. Biorxiv 2021. [Google Scholar] [CrossRef]
- Mertz, K.H.; Reitelseder, S.; Bechshoeft, R.; Bulow, J.; Højfeldt, G.; Jensen, M.; Schacht, S.R.; Lind, M.V.; A Rasmussen, M.; Mikkelsen, U.R.; et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 790–800. [Google Scholar] [CrossRef] [PubMed]
- DHS, The Danish Healthcare System. 2003: The Joint Public Health Portal, Sundhed.Dk. Available online: https://www.sundhed.dk (accessed on 15 December 2020).
- Mooradian, A.D.; Haas, M.J.; Wehmeier, K.R.; Wong, N.C. Obesity-related Changes in High-density Lipoprotein Metabolism. Obesity 2008, 16, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Genest, J. Effect of Obesity on High-density Lipoprotein Metabolism. Obesity 2007, 15, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Vertommen, J.; De Leeuw, I.H. The in vitro oxidizability of lipoprotein particles in obese and non-obese subjects. Atherosclerosis 1998, 137, S39–S44. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2019, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Trimigno, A.; Aru, V.; Rasmussen, M.A.; Khakimov, B.; Engelsen, S.B. Influence of Age, Sex, and Diet on the Human Fecal Metabolome Investigated by 1H NMR Spectroscopy. J. Proteome Res. 2021, 20, 3642–3653. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.; Høstmark, A.T.; Spydevold, Ø/. Plasma lipoprotein distribution, faecal cholesterol excretion, and activities of lipoprotein lipase, hepatic lipase and lecithin:cholesterol acyltransferase in rats fed diets rich in sucrose or sunflower oil. Acta Physiol. Scand. 1985, 125, 609–617. [Google Scholar] [CrossRef]
- Wilson, P.; D’Agostino, R.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrinilogy 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut Microbiota 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med.-Open 2018, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, G.M.; Coffey, V.G.; Niere, J.O.; Hawley, J.; Adams, M.J. Spectroscopic correlation analysis of NMR-based metabonomics in exercise science. Anal. Chim. Acta 2009, 652, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deda, O.; Gika, H.; Panagoulis, T.; Taitzoglou, I.; Raikos, N.; Theodoridis, G. Impact of exercise on fecal and cecal metabolome over aging: A longitudinal study in rats. Bioanalytical 2017, 9, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 2021, 29, 394–407. [Google Scholar] [CrossRef]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, É.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolism 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Bogdanffy, M.S.; Sarangapani, R.; Plowchalk, D.R.; Jarabek, A.; E Andersen, M. A biologically based risk assessment for vinyl acetate-induced cancer and noncancer inhalation toxicity. Toxicol. Sci. 1999, 51, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayashima, T.; Katayama, T. Oxalic acid is available as a natural antioxidant in some systems. Biochim. Et Biophys. Acta-Gen. Sub. 2002, 1573, 1–3. [Google Scholar] [CrossRef]
- Holt, P.R. Intestinal Malabsorption in the Elderly. Dig. Dis. 2007, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Seidell, J.C.; E Meinders, A. [The value and limitations of the body mass index (BMI) in the assessment of the health risks of overweight and obesity]. Ned. Tijdschr. voor Geneeskd. 2004, 148, 2379–2382. [Google Scholar]
- Salazar, N.; González, S.; Nogacka, A.M.; Rios-Covián, D.; Arboleya, S.; Gueimonde, M.; Reyes-Gavilán, C.G.D.L. Microbiome: Effects of Ageing and Diet. Microbiota Curr. Res. Emerg. Trends 2019, 36, 33–61. [Google Scholar]
- Liang, S.; Hou, Z.; Li, X.; Wang, J.; Cai, L.; Zhang, R.; Li, J. The fecal metabolome is associated with gestational diabetes mellitus. RSC Adv. 2019, 9, 29973–29979. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Men, L.; Pi, Z.; Wei, M.; Song, F.; Zhao, C.; Liu, Z. Fecal Metabolomics of Type 2 Diabetic Rats and Treatment with Gardenia jasminoides Ellis Based on Mass Spectrometry Technique. J. Agric. Food Chem. 2018, 66, 1591–1599. [Google Scholar] [CrossRef]
- Jiang, J.; Nilsson-Ehle, P.; Xu, N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Pasiakos, S.M.; Lieberman, H.R.; Fulgoni, I.V.L. Higher-Protein Diets Are Associated with Higher HDL Cholesterol and Lower BMI and Waist Circumference in US Adults. J. Nutr. 2015, 145, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Liu, Z.; Liu, P. HDL cholesterol in cardiovascular diseases: The good, the bad, and the ugly? Int. J. Cardiol. 2013, 168, 3157–3159. [Google Scholar] [CrossRef]
- Stadler, J.; Lackner, S.; Mörkl, S.; Trakaki, A.; Scharnagl, H.; Borenich, A.; Wonisch, W.; Mangge, H.; Zelzer, S.; Meier-Allard, N.; et al. Obesity Affects HDL Metabolism, Composition and Subclass Distribution. Biomedical 2021, 9, 242. [Google Scholar]
- Schmedes, M.S.; Yde, C.C.; Svensson, U.; Håkansson, J.; Baby, S.; Bertram, H.C. Impact of a 6-week very low-calorie diet and weight reduction on the serum and fecal metabolome of overweight subjects. Eur. Food Res. Technol. 2015, 240, 583–594. [Google Scholar] [CrossRef]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef]
- Bechshøft, R.L.; Reitelseder, S.; Højfeldt, G.; Castro-Mejía, J.L.; Khakimov, B.; Bin Ahmad, H.F.; Kjær, M.; Engelsen, S.B.; Johansen, S.M.B.; Rasmussen, M.A.; et al. Counteracting Age-related Loss of Skeletal Muscle Mass: A clinical and ethnological trial on the role of protein supplementation and training load (CALM Intervention Study): Study protocol for a randomized controlled trial. Trials 2016, 17, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Thatte, U.; Puri, K.; Suresh, K.; Gogtay, N. Declaration of Helsinki, 2008: Implications for stakeholders in research. J. Postgrad. Med. 2009, 55, 131–134. [Google Scholar] [CrossRef]
- Khakimov, B.; Motawia, M.S.; Bak, S.; Engelsen, S.B. The use of trimethylsilyl cyanide derivatization for robust and broad-spectrum high-throughput gas chromatography–mass spectrometry based metabolomics. Anal. Bioanal. Chem. 2013, 405, 9193–9205. [Google Scholar] [CrossRef]
- Khakimov, B.; Mobaraki, N.; Trimigno, A.; Aru, V.; Engelsen, S.B. Signature Mapping (SigMa): An efficient approach for processing complex human urine 1H NMR metabolomics data. Anal. Chim. Acta 2020, 1108, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Michel, N.; Akoka, S. The application of the ERETIC method to 2D-NMR. J. Magn. Reson. 2004, 168, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Poulsen, S.K.; Savorani, F.; Acar, E.; Gürdeniz, G.; Larsen, T.M.; Astrup, A.; Dragsted, L.O.; Engelsen, S.B. New Nordic Diet versus Average Danish Diet: A Randomized Controlled Trial Revealed Healthy Long-Term Effects of the New Nordic Diet by GC–MS Blood Plasma Metabolomics. J. Proteome Res. 2016, 15, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Smilde, A.K.; Jan, V.D.G.; Hoefsloot, H.C.J.; Lamers, R.-J.A.N.; Van Der Greef, J.; E Timmerman, M. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Ståhle, L.; Wold, S. Partial least squares analysis with cross-validation for the two-class problem: A Monte Carlo study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Chumbley, J.R.; Friston, K. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage 2009, 44, 62–70. [Google Scholar] [CrossRef] [PubMed]




| Metabolite | CVD Risk Factor | p-Value | Effect Size (%) | Fold Change(MH/ML) |
|---|---|---|---|---|
| butyric acid | HDLchol | 0.038 | 4.02 | 1.28 |
| LDLchol/HDLchol | 0.033 | 4.20 | 0.78 | |
| VLDLchol/HDLchol | 0.044 | 3.73 | 0.79 | |
| isovaleric acid | HDLchol | 0.001 | 11.08 | 0.80 |
| VLDLchol | 0.013 | 5.63 | 1.29 | |
| leucine | LDLchol | 0.030 | 4.55 | 0.85 |
| total chol/HDL-Apo A | 0.016 | 5.28 | 0.84 | |
| tg | 0.039 | 4.63 | 0.76 | |
| valine | total chol/HDLchol | 0.003 | 9.20 | 0.68 |
| total chol/HDL-Apo A | 0.022 | 4.80 | 0.84 | |
| alanine | total chol/HDLchol | 0.002 | 9.90 | 0.58 |
| glutamic acid | total chol/HDLchol | 0.020 | 5.02 | 0.67 |
| LDLchol | 0.011 | 6.17 | 0.67 | |
| LDLchol/HDLchol | 0.025 | 4.68 | 0.68 | |
| phenylalanine | LDLchol | 0.019 | 5.30 | 0.84 |
| total chol/HDL-Apo A | 0.011 | 5.90 | 0.88 | |
| proline | tg | 0.041 | 4.54 | 0.69 |
| aspartic acid | tg | 0.004 | 8.67 | 0.48 |
| methanol | total chol/HDLchol | 0.017 | 5.27 | 0.73 |
| LDLchol | 0.020 | 5.22 | 0.66 | |
| glycine | total chol/HDL-Apo A | 0.020 | 4.96 | 0.85 |
| lysine | total chol/HDL-Apo A | 0.040 | 3.87 | 0.85 |
| methionine | total chol/HDL-Apo A | 0.031 | 4.27 | 0.78 |
| LDLchol/HDLchol | 0.038 | 4.01 | 0.80 | |
| lactic acid | total chol/HDL-Apo A | 0.045 | 3.71 | 0.74 |
| isoleucine | total chol/HDL-Apo A | 0.023 | 4.74 | 0.84 |
| uracil | total chol/HDL-Apo A | 0.013 | 5.67 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, M.; Trimigno, A.; Castro-Mejía, J.L.; Reitelseder, S.; Bülow, J.; Bechshøft, R.L.; Nielsen, D.S.; Holm, L.; Engelsen, S.B.; Khakimov, B. Human Fecal Metabolome Reflects Differences in Body Mass Index, Physical Fitness, and Blood Lipoproteins in Healthy Older Adults. Metabolites 2021, 11, 717. https://doi.org/10.3390/metabo11110717
Cui M, Trimigno A, Castro-Mejía JL, Reitelseder S, Bülow J, Bechshøft RL, Nielsen DS, Holm L, Engelsen SB, Khakimov B. Human Fecal Metabolome Reflects Differences in Body Mass Index, Physical Fitness, and Blood Lipoproteins in Healthy Older Adults. Metabolites. 2021; 11(11):717. https://doi.org/10.3390/metabo11110717
Chicago/Turabian StyleCui, Mengni, Alessia Trimigno, Josue L. Castro-Mejía, Søren Reitelseder, Jacob Bülow, Rasmus Leidesdorff Bechshøft, Dennis Sandris Nielsen, Lars Holm, Søren Balling Engelsen, and Bekzod Khakimov. 2021. "Human Fecal Metabolome Reflects Differences in Body Mass Index, Physical Fitness, and Blood Lipoproteins in Healthy Older Adults" Metabolites 11, no. 11: 717. https://doi.org/10.3390/metabo11110717