Application of Biocompatible Noble Metal Film Materials to Medical Implants: TiNi Surface Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. MOCVD Precursors
2.3. Noble Metal Films Deposition
2.4. Characterization Methods
2.5. Differential Scanning Calorimetry
2.6. Cytotoxicity Testing
2.7. Statistical Analysis
2.8. Biological Extracts Studies
3. Results and Discussion
3.1. Composition and Microstructure of Compact Iridium Sublayers on Si(100) Substrates
3.2. Deposition of Protective Noble Metal Sublayers onto TiNi Substrates
3.3. Deposition of Developed Ir onto TiNi Substrates Coated with Noble Metal Sublayers
3.4. DSC Investigation of TiNi Coated with Noble Metal Films
3.5. Biological Characteristics of TiNi Coated with Noble Metal Films
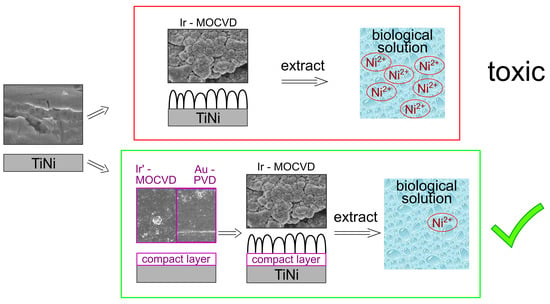
4. Conclusions
- A new MOCVD approach to obtaining compact protective iridium layers (Ir’) is demonstrated. In fact, the usage of the Ir(CO)2(acac) precursor at relatively low temperature of 280 °C in a reducing atmosphere under VUV radiation ensure the emergence of multiple nucleation centers.
- The application of a compact protective Ir’ or Au sublayer as thin as <100 nm effectively prevent the release of nickel and titanium from TiNi. In fact, the concentrations of metals in the culture medium extracts obtained using Au/TiNi and Ir’/TiNi are more than two times lower than for bare TiNi.
- Application of new Ir’ protective layer is the most effective due to the absence of cracks and clearances (Ni concentration decrease by an order of magnitude, up to 0.005 µg/mL).
- Cytotoxicity test (MAN-1 cells) confirm the high biocompatibility of the proposed noble metal film materials (cell viability ≥ 97%). Moreover, Au sublayer increases cell proliferation (cell viability ≥ 120%).
- The protective functions of the sublayers are sufficiently preserved after the deposition of an Ir coating with a developed morphology on them by MOCVD in an oxidizing atmosphere at 350 °C. The application of the latter type of Ir coating previously stimulated increased nickel release, and accordingly, a strong cytotoxic effect on MAN-1 cells. In this work, such effects were prevented, since Ir/Ir’/TiNi and Ir/Au/TiNi sample extracts are characterized by a cell viability ≥99% with the nickel concentration <0.1 µg/mL.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [Green Version]
- Valiev, R.Z.; Prokofiev, E.A.; Kazarinov, N.A.; Raab, G.I.; Minasov, T.B.; Stráský, J. Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices. Materials 2020, 13, 967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unune, D.R.; Brown, G.R.; Reilly, G.C. Thermal Based Surface Modification Techniques for Enhancing the Corrosion and Wear Resistance of Metallic Implants: A Review. Vacuum 2022, 203, 111298. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and Processing of NiTi Implants: A Review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Sargeant, T.D.; Stupp, S.I.; Dunand, D.C. Porous NiTi for Bone Implants: A Review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zeng, Q.; Fu, T. An Updated Review on TiNi Alloy for Biomedical Applications. Corros. Rev. 2019, 37, 539–552. [Google Scholar] [CrossRef]
- Yeung, K.W.K.; Cheung, K.M.C.; Lu, W.W.; Chung, C.Y. Optimization of Thermal Treatment Parameters to Alter Austenitic Phase Transition Temperature of NiTi Alloy for Medical Implant. Mater. Sci. Eng. A 2004, 383, 213–218. [Google Scholar] [CrossRef]
- Velmurugan, C.; Senthilkumar, V.; Dinesh, S.; Arulkirubakaran, D. Review on Phase Transformation Behavior of NiTi Shape Memory Alloys. Mater. Today Proc. 2018, 5, 14597–14606. [Google Scholar] [CrossRef]
- Chan, W.-S.; Gulati, K.; Peters, O.A. Advancing Nitinol: From Heat Treatment to Surface Functionalization for Nickel–Titanium (NiTi) Instruments in Endodontics. Bioact. Mater. 2023, 22, 91–111. [Google Scholar] [CrossRef]
- Lex, J.R.; Koucheki, R.; Stavropoulos, N.A.; Di Michele, J.; Toor, J.S.; Tsoi, K.; Ferguson, P.C.; Turcotte, R.E.; Papagelopoulos, P.J. Megaprosthesis Anti-Bacterial Coatings: A Comprehensive Translational Review. Acta Biomater. 2022, 140, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Sarao, T.P.S.; Singh, H.; Singh, H. Enhancing Biocompatibility and Corrosion Resistance of Ti-6Al-4V Alloy by Surface Modification Route. J. Therm. Spray Technol. 2018, 27, 1388–1400. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive Coating as a Surface Modification Technique for Biocompatible Metallic Implants: A Review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Na, X.; Jijiang, F.; Lingzhou, Z.; Chu, P.K.; Huo, K. Biofunctional Elements Incorporated Nano/Microstructured Coatings on Titanium Implants with Enhanced Osteogenic and Antibacterial Performance. Adv. Healthc. Mater 2020, 9, 2000681. [Google Scholar]
- Zhao, T.; Yang, R.; Zhong, C.; Li, Y.; Xiang, Y. Effective Inhibition of Nickel Release by Tantalum-Implanted TiNi Alloy and Its Cyto-Compatibility Evaluation in Vitro. J. Mater. Sci. 2011, 46, 2529–2535. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium Ionic Species from Degradation of Ti-6Al-4V Metallic Implants: In Vitro Cytotoxicity and Speciation Evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, H.; Muhamad, N.; Sulong, A.B.; Ahmadipour, M. Recent Advances on Biofunctionalization of Metallic Substrate Using Ceramic Coating: How Far Are We from Clinically Stable Implant? J. Taiwan Inst. Chem. Eng. 2021, 118, 254–270. [Google Scholar] [CrossRef]
- Beig, B.; Liaqat, U.; Niazi, M.F.K.; Douna, I.; Zahoor, M.; Niazi, M.B.K. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Kostelac, L.; Pezzato, L.; Settimi, A.G.; Franceschi, M.; Gennari, C.; Brunelli, K.; Rampazzo, C.; Dabalà, M. Investigation of Hydroxyapatite (HAP) Containing Coating on Grade 2 Titanium Alloy Prepared by Plasma Electrolytic Oxidation (PEO) at Low Voltage. Surf. Interfaces 2022, 30, 101888. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A Sol–Gel Based Silver Nanoparticle/Polytetrafluorethylene (AgNP/PTFE) Coating with Enhanced Antibacterial and Anti-Corrosive Properties. Appl. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]
- Liu, L.; Peng, W.; Zhang, X.; Peng, J.; Liu, P.; Shen, J. Rational Design of Phosphonate/Quaternary Amine Block Polymer as an High-Efficiency Antibacterial Coating for Metallic Substrates. J. Mater. Sci. Technol. 2021, 62, 96–106. [Google Scholar] [CrossRef]
- Voicu, G.; Miu, D.; Ghitulica, C.D.; Jinga, S.I.; Nicoara, A.I.; Busuioc, C.; Holban, A.M. Co Doped ZnO Thin Films Deposited by Spin Coating as Antibacterial Coating for Metallic Implants. Ceram. Int. 2020, 46, 3904–3911. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Z.; Wang, Q.; Zu, S.; Liu, M.; Yu, Z.; Zhang, Z.; Ren, L. Corrosion Resistance and Biocompatibility of Graphene Oxide Coating on the Surface of the Additively Manufactured NiTi Alloy. Prog. Org. Coat. 2022, 164, 106722. [Google Scholar] [CrossRef]
- Gunputh, U.F.; Le, H. A Review of In-Situ Grown Nanocomposite Coatings for Titanium Alloy Implants. J. Compos. Sci. 2020, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The Use of Noble Metal Coatings and Nanoparticles for the Modification of Medical Implant Materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble Metal-Based Nanomaterials as Antibacterial Agents. J. Alloys Compd. 2022, 904, 164091. [Google Scholar] [CrossRef]
- Dowling, D.; Betts, A..; Pope, C.; McConnell, M..; Eloy, R.; Arnaud, M. Anti-Bacterial Silver Coatings Exhibiting Enhanced Activity through the Addition of Platinum. Surf. Coat. Technol. 2003, 163–164, 637–640. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Bae, I.-H.; Lee, K.-G.; Hwang, H.-S.; Lee, K.-H.; Koh, J.-T.; Cho, J.-H. Antibacterial Effect of Silver-Platinum Coating for Orthodontic Appliances. Angle Orthod. 2012, 82, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowska-Szczupak, A.; Paszkiewicz, O.; Michalkiewicz, B.; Kamińska, A.; Wróbel, R.J. Fabrication of Antibacterial Metal Surfaces Using Magnetron-Sputtering Method. Materials 2021, 14, 7301. [Google Scholar] [CrossRef]
- Cai, T.; Fang, G.; Tian, X.; Yin, J.J.; Chen, C.; Ge, C. Optimization of Antibacterial Efficacy of Noble-Metal-Based Core-Shell Nanostructures and Effect of Natural Organic Matter. ACS Nano 2019, 13, 12694–12702. [Google Scholar] [CrossRef]
- Ramteke, L.; Gawali, P.; Jadhav, B.L.; Chopade, B.A. Comparative Study on Antibacterial Activity of Metal Ions, Monometallic and Alloy Noble Metal Nanoparticles Against Nosocomial Pathogens. Bionanoscience 2020, 10, 1018–1036. [Google Scholar] [CrossRef]
- Abuayyash, A.; Ziegler, N.; Meyer, H.; Meischein, M.; Sengstock, C.; Moellenhoff, J.; Rurainsky, C.; Heggen, M.; Garzón-Manjón, A.; Scheu, C.; et al. Enhanced Antibacterial Performance of Ultrathin Silver/Platinum Nanopatches by a Sacrificial Anode Mechanism. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102126. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef] [Green Version]
- Breisch, M.; Loza, K.; Pappert, K.; Rostek, A.; Rurainsky, C.; Tschulik, K.; Heggen, M.; Epple, M.; Tiller, J.C.; Schildhauer, T.A.; et al. Enhanced Dissolution of Silver Nanoparticles in a Physical Mixture with Platinum Nanoparticles Based on the Sacrificial Anode Effect. Nanotechnology 2020, 31, 055703. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Chepeleva, E.V.; Vasilieva, M.B.; Nasimov, D.A.; Maksimovskii, E.A.; Tsygankova, A.R.; Basova, T.V.; Sergeevichev, D.S.; Morozova, N.B. Noble Metals for Modern Implant Materials: MOCVD of Film Structures and Cytotoxical, Antibacterial, and Histological Studies. Biomedicines 2021, 9, 851. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Sergeevichev, D.S.; Guselnikova, T.Y.; Zheravin, A.A.; Nasimov, D.A.; Vasilieva, M.B.; Chepeleva, E.V.; Saprykin, A.I.; Basova, T.V.; et al. Biological Studies of New Implant Materials Based on Carbon and Polymer Carriers with Film Heterostructures Containing Noble Metals. Biomedicines 2022, 10, 2230. [Google Scholar] [CrossRef] [PubMed]
- Köller, M.; Sengstock, C.; Motemani, Y.; Khare, C.; Buenconsejo, P.J.S.; Geukes, J.; Schildhauer, T.A.; Ludwig, A. Antibacterial Activity of Microstructured Ag/Au Sacrificial Anode Thin Films. Mater. Sci. Eng. C 2015, 46, 276–280. [Google Scholar] [CrossRef]
- Köller, M.; Bellova, P.; Javid, S.M.; Motemani, Y.; Khare, C.; Sengstock, C.; Tschulik, K.; Schildhauer, T.A.; Ludwig, A. Antibacterial Activity of Microstructured Sacrificial Anode Thin Films by Combination of Silver with Platinum Group Elements (Platinum, Palladium, Iridium). Mater. Sci. Eng. C 2017, 74, 536–541. [Google Scholar] [CrossRef] [PubMed]
- El Arrassi, A.; Bellova, P.; Javid, S.M.; Motemani, Y.; Khare, C.; Sengstock, C.; Köller, M.; Ludwig, A.; Tschulik, K. A Unified Interdisciplinary Approach to Design Antibacterial Coatings for Fast Silver Release. ChemElectroChem 2017, 4, 1975–1983. [Google Scholar] [CrossRef]
- Abuayyash, A.; Ziegler, N.; Gessmann, J.; Sengstock, C.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Antibacterial Efficacy of Sacrifical Anode Thin Films Combining Silver with Platinum Group Elements within a Bacteria-Containing Human Plasma Clot. Adv. Eng. Mater. 2018, 20, 1700493. [Google Scholar] [CrossRef]
- Groessner-Schreiber, B.; Neubert, A.; Müller, W.-D.; Hopp, M.; Griepentrog, M.; Lange, K.-P. Fibroblast Growth on Surface-Modified Dental Implants: An in Vitro Study. J. Biomed. Mater. Res. Part A 2003, 64, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.; Ahmad, M. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-Scale Modification of Titanium Implant Surfaces to Enhance Osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Koretskaya, T.P.; Korolkov, I.V.; Chepeleva, E.V.; Asanov, I.P.; Tsygankova, A.R.; Maksimovskii, E.A.; Marchenko, E.S.; Lantsukhay, Y.A.; et al. MOCVD of Noble Metal Film Materials for Medical Implants: Microstructure and Biocompatibility of Ir and Au/Ir Coatings on TiNi. Coatings 2021, 11, 638. [Google Scholar] [CrossRef]
- Gunther, V.; Marchenko, E.; Chekalkin, T.; Baigonakova, G.; Kang, J.; Kim, J.; Klopotov, A. Study of Structural Phase Transitions in Quinary TiNi(MoFeAg)-Based Alloys. Mater. Res. Express 2017, 4, 105702. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Ilyin, I.Y.; Zelenina, L.N.; Sysoev, S.V.; Morozova, N.B. Thermodynamic Study of Volatile Iridium (I) Complexes with 1,5-Cyclooctadiene and Acetylacetonato Derivatives: Effect of (O,O) and (O,N) Coordination Sites. J. Chem. Thermodyn. 2019, 133, 194–201. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Kuratieva, N.V.; Morozova, N.B. Crystal Structure of (Acetylacetonato) (Dicarbonyl)Iridium(I). J. Struct. Chem. 2009, 50, 574–576. [Google Scholar] [CrossRef]
- Karakovskaya, K.I.; Dorovskikh, S.I.; Vikulova, E.S.; Ilyin, I.Y.; Zherikova, K.V.; Basova, T.V.; Morozova, N.B. Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine. Coatings 2021, 11, 78. [Google Scholar] [CrossRef]
- Zamchiy, A.O.; Baranov, E.A.; Konstantinov, V.O.; Lunev, N.A.; Sakhapov, S.Z.; Korolkov, I.V.; Volodin, V.A. Activation Energy of Gold-Induced Crystallization of Amorphous Silicon Suboxide Films. Mater. Lett. 2022, 323, 132566. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Kraus, W.; Nolze, G. POWDER CELL—A Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-ray Powder Patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray Photoelectron Spectroscopy: Towards Reliable Binding Energy Referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-ray Photoelectron Spectra of Ir, IrO2 and IrCl3 Revisited. Surf. Interface Anal. 2017, 49, 794–799. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical Properties, Degradation Performance and Cytotoxicity of Mg-Zn-Ca Biomedical Alloys with Different Compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Grigor’eva, E.V.; Malankhanova, T.B.; Surumbayeva, A.; Pavlova, S.V.; Minina, J.M.; Kizilova, E.A.; Suldina, L.A.; Morozova, K.N.; Kiseleva, E.; Sorokoumov, E.D.; et al. Generation of GABAergic Striatal Neurons by a Novel IPSC Differentiation Protocol Enabling Scalability and Cryopreservation of Progenitor Cells. Cytotechnology 2020, 72, 649–663. [Google Scholar] [CrossRef]
- Gouveia, S.T.; Silva, F.V.; Costa, L.M.; Nogueira, A.R.A.; Nóbrega, J.A. Determination of Residual Carbon by Inductively-Coupled Plasma Optical Emission Spectrometry with Axial and Radial View Configurations. Anal. Chim. Acta 2001, 445, 269–275. [Google Scholar] [CrossRef]
- de Souza, R.M.; Leocádio, L.G.; da Silveira, C.L.P. ICP OES Simultaneous Determination of Ca, Cu, Fe, Mg, Mn, Na, and P in Biodiesel by Axial and Radial Inductively Coupled Plasma-Optical Emission Spectrometry. Anal. Lett. 2008, 41, 1615–1622. [Google Scholar] [CrossRef]
- Abelson, J.R.; Girolami, G.S. New Strategies for Conformal, Superconformal, and Ultrasmooth Films by Low Temperature Chemical Vapor Deposition. J. Vac. Sci. Technol. A 2020, 38, 030802. [Google Scholar] [CrossRef] [Green Version]
- Igumenov, I.K.; Gelfond, N.V.; Morozova, N.B.; Nizard, H. Overview of Coating Growth Mechanisms in MOCVD Processes as Observed in Pt Group Metals. Chem. Vap. Depos. 2007, 13, 633–637. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Korolkov, I.V.; Maksimovskiy, E.A.; Kal’nyi, D.B.; Morozova, N.B. Microstructure of Iridium Enriched PtxIr(1–x) Films Prepared by Chemical Vapor Deposition. J. Struct. Chem. 2021, 62, 1447–1456. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Kal’nyi, D.B.; Shubin, Y.V.; Kokovkin, V.V.; Morozova, N.B.; Hassan, A.; Basova, T.V. Metal Ir Coatings on Endocardial Electrode Tips, Obtained by MOCVD. Appl. Surf. Sci. 2017, 425, 1052–1058. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Chan, Y.L.; Ho, J.P.Y.; Chung, C.Y.; Chu, P.K.; Chu, C.L.; Yeung, K.W.K.; Lu, W.W.; Cheung, K.M.C.; et al. Nickel Release Behavior, Cytocompatibility, and Superelasticity of Oxidized Porous Single-Phase NiTi. J. Biomed. Mater. Res. Part A 2007, 81, 948–955. [Google Scholar] [CrossRef]
- Tanaka, S.; Nomura, N.; Nishioka, T.; Hirotsu, M.; Nakajima, H. Synthesis of Iron(III)-Carbonyl Complex with Variable Wavelength Range for CO Release Depending on Protonation and Deprotonation of Axial Phosphorous Ligands. J. Organomet. Chem. 2021, 943, 121843. [Google Scholar] [CrossRef]
- Wong, M.H.; Cheng, F.T.; Pang, G.K.H.; Man, H.C. Characterization of Oxide Film Formed on NiTi by Laser Oxidation. Mater. Sci. Eng. A 2007, 448, 97–103. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Dan, A.; Bijalwan, P.K.; Pathak, A.S.; Bhagat, A.N. A Review on Physical Vapor Deposition-Based Metallic Coatings on Steel as an Alternative to Conventional Galvanized Coatings. J. Coat. Technol. Res. 2022, 19, 403–438. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, J.; Han, G.H.; Guo, W.; Hong, S.; Park, H.S.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Dendritic Gold-Supported Iridium/Iridium Oxide Ultra-Low Loading Electrodes for High-Performance Proton Exchange Membrane Water Electrolyzer. Appl. Catal. B Environ. 2021, 283, 119596. [Google Scholar] [CrossRef]
- Marchenko, E.; Baigonakova, G.; Yasenchuk, Y. Gradient Crystalline Coating on a Biomedical TiNi Alloy Prepared by Magnetron Sputtering and Annealing. Vacuum 2020, 181, 109652. [Google Scholar] [CrossRef]
- Jia, S.; Ge, S.; Fan, X.; Leong, K.W.; Ruan, J. Promoting Reactive Oxygen Species Generation: A Key Strategy in Nanosensitizer-Mediated Radiotherapy. Nanomedicine 2021, 16, 759–778. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. In Reactive Oxygen Species (ROS) in Living Cells; InTech: Rang-Du-Fliers, France, 2018. [Google Scholar]
- Carvajal Carvajal, C. Reactive Oxygen Species: Training, Function and Oxidative Stress. Rev. Med. Leg. Costa Rica 2019, 36, 91–100. [Google Scholar]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]














| Sample | Sublayer | Developed Coating | Characterization | Layer Thickness (nm) | ||
|---|---|---|---|---|---|---|
| Metal | Deposition Method | Metal | Deposition Method | |||
| Au/TiNi | gold | PVD, 300 °C | - | - | XRD, SEM, XPS, cytotoxicity test *, Ni release * | 90 nm (model layer on Si) 70 nm (estimated from XPS) |
| Ir’/TiNi | iridium | MOCVD, Ir(CO)2(acac) + H2, VUV, 280 °C | - | - | XRD, SEM, cross-section SEM, XPS, DSC *, cytotoxicity test *, Ni release * | 85 nm (model layer on Si); 50 nm (cross-section SEM) |
| Ir/TiNi | - | - | iridium | MOCVD, Ir(cod)(acac) + O2, 350 °C | Cross-section SEM (this work); XRD, SEM, cytotoxicity test *, Ni release * [49] | 800–1000 nm (cross-section SEM) |
| Ir/Au/TiNi | gold | PVD, 300 °C | iridium | XRD, SEM, XPS cytotoxicity test *, Ni release * | Ir: 800–900 nm Au: 60–80 nm (estimated from XPS) | |
| Ir/Ir’/TiNi | iridium | MOCVD, Ir(acac)(CO)2 + H2, VUV, 280 °C | iridium | XRD, SEM, cross-section SEM, XPS, DSC *, cytotoxicity test *, Ni release * | Ir: 1000 nm Ir’: 50 nm (cross-section SEM) | |
| No | Tdep. (°C) | Tevap. (°C) | τ (min) | VUV | Growth Rate (nm/min) | Layer Thickness (nm) |
|---|---|---|---|---|---|---|
| 1 | 280 | 80 | 60 | − | 0.67 | 40 |
| 2 | 80 | 60 | + | 0.83 | 50 | |
| 3 | 60 | 120 | + | 0.71 | 85 | |
| 4 | 300 | 60 | 120 | + | 0.54 | 60 |
| Sample Group | pH | Ni Content (µg/mL) | Ti Content (µg/mL) |
|---|---|---|---|
| Control (culture medium) | 7.70 | n.d. (0.001) * | n.d. (0.0005) * |
| TiNi | 7.63 | 0.056 | 0.0075 |
| Ir’/TiNi | 7.74 | 0.005 | 0.0017 |
| Ir/Ir’/TiNi | 7.74 | 0.040 | 0.0082 |
| Au/TiNi | 7.75 | 0.027 | 0.0036 |
| Ir/Au/TiNi | 7.85 | 0.090 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikulova, E.S.; Karakovskaya, K.I.; Korolkov, I.V.; Koretskaya, T.P.; Chepeleva, E.V.; Kuz’min, N.B.; Fedorenko, A.D.; Pischur, D.P.; Guselnikova, T.Y.; Maksimovskii, E.A.; et al. Application of Biocompatible Noble Metal Film Materials to Medical Implants: TiNi Surface Modification. Coatings 2023, 13, 222. https://doi.org/10.3390/coatings13020222
Vikulova ES, Karakovskaya KI, Korolkov IV, Koretskaya TP, Chepeleva EV, Kuz’min NB, Fedorenko AD, Pischur DP, Guselnikova TY, Maksimovskii EA, et al. Application of Biocompatible Noble Metal Film Materials to Medical Implants: TiNi Surface Modification. Coatings. 2023; 13(2):222. https://doi.org/10.3390/coatings13020222
Chicago/Turabian StyleVikulova, Evgeniia S., Ksenya I. Karakovskaya, Ilya V. Korolkov, Tatyana P. Koretskaya, Elena V. Chepeleva, Nikolay B. Kuz’min, Anastasiya D. Fedorenko, Denis P. Pischur, Tatiana Ya. Guselnikova, Eugene A. Maksimovskii, and et al. 2023. "Application of Biocompatible Noble Metal Film Materials to Medical Implants: TiNi Surface Modification" Coatings 13, no. 2: 222. https://doi.org/10.3390/coatings13020222