A Prunus avium L. Infusion Inhibits Sugar Uptake and Counteracts Oxidative Stress-Induced Stimulation of Glucose Uptake by Intestinal Epithelial (Caco-2) Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Sample Preparation
2.2. Caco-2 Cell Culture
2.3. Cell Treatments
2.4. Determination of 3H-Deoxy-D-Glucose and 14C-Fructose Apical Uptake
2.5. Evaluation of the Apical-to-Basolateral Transepithelial Transport (Apparent Permeability)
2.6. Real-Time Quantitative Reverse Transcription PCR
2.7. Total Protein Determination
2.8. Analysis of Phenolic Compounds by UHPLC-ESI-QTOF-MS
2.9. Statistical Analysis
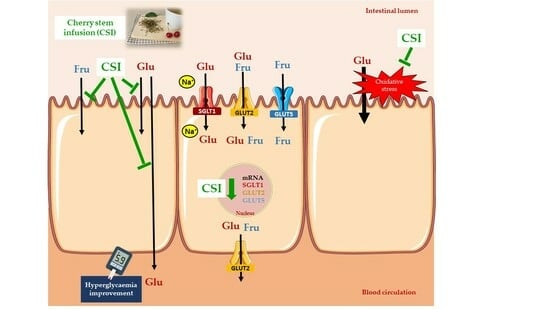
3. Results and Discussion
3.1. Cherry Stem Infusion Reduces 3H-DG and 14C-FRU Apical Uptake by Caco-2 Cells
3.2. Cherry Stem Infusion Reduces the Apical-to-Basolateral Papp to 3H-DG in Caco-2 Cells
3.3. Cherry Stem Infusion Affects the Expression of Glucose and Fructose Intestinal Transporters
3.4. The Inhibitory Effect of the Cherry Stem Infusion upon the Uptake of 3H-DG Is Related to Its Antioxidant Activity
3.5. Quantification of Phenolic Compounds in the Cherry Stem Infusion by UHPLC-ESI-QTOF-MS
3.6. The Antidiabetic Potential of Cherry Stem Infusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules 2022, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.; Alvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Andrade, N.; Moreira, A.; Cifuentes, A.; Martel, F.; Oliveira, M.; Ibanez, E. Cherry stem infusions: Antioxidant potential and phenolic profile by UHPLC-ESI-QTOF-MS. Food Funct. 2020, 11, 3471–3482. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Babota, M.; Vostinaru, O.; Paltinean, R.; Mihali, C.; Dias, M.I.; Barros, L.; Ferreira, I.; Mocan, A.; Crisan, O.; Nicula, C.; et al. Chemical Composition, Diuretic, and Antityrosinase Activity of Traditionally Used Romanian Cerasorum stipites. Front. Pharmacol. 2021, 12, 647947. [Google Scholar] [CrossRef]
- Bursal, E.; Köksal, E.; Gülçin, I.; Bilsel, G.; Gören, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid Oxidation Products in the Pathogenesis of Inflammation-related Gut Diseases. Curr. Med. Chem. 2018, 25, 1311–1326. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells–Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Silva, C.; Martel, F. The effect of oxidative stress upon intestinal sugar transport: An in vitro study using human intestinal epithelial (Caco-2) cells. Toxicol. Res. 2018, 7, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Packard, C.J.; Boren, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Te Morenga, L. Sugar and Type 2 diabetes. Br. Med. Bull. 2016, 120, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Merino, B.; Fernandez-Diaz, C.M.; Cozar-Castellano, I.; Perdomo, G. Intestinal Fructose and Glucose Metabolism in Health and Disease. Nutrients 2019, 12, 94. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Aranias, T.; Viel, T.; Chateau, D.; Le Gall, M.; Waligora-Dupriet, A.J.; Melchior, C.; Rouxel, O.; Kapel, N.; Gourcerol, G.; et al. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol. Metab. 2017, 6, 61–72. [Google Scholar] [CrossRef]
- Guney, C.; Bal, N.B.; Akar, F. The impact of dietary fructose on gut permeability, microbiota, abdominal adiposity, insulin signaling and reproductive function. Heliyon 2023, 9, e18896. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Marques, C.; Andrade, S.; Silva, C.; Rodrigues, I.; Guardao, L.; Guimaraes, J.T.; Keating, E.; Calhau, C.; Martel, F. Effect of chrysin on changes in intestinal environment and microbiome induced by fructose-feeding in rats. Food Funct. 2019, 10, 4566–4576. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Andrade, N.; Machado, S.; Costa, A.S.G.; Puga, H.; Oliveira, M.; Martel, F.; Alves, R.C. Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale. Foods 2022, 11, 1671. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Aires, A.; Dias, C.; Carvalho, R.; Saavedra, M.J. Analysis of glycosylated flavonoids extracted from sweet-cherry stems, as antibacterial agents against pathogenic Escherichia coli isolates. Acta Biochim. Pol. 2017, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.; Barros, L.; Duenas, M.; Calhelha, R.C.; Queiroz, M.J.; Santos-Buelga, C.; Ferreira, I.C. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Goncalves, A.C.; Alves, G.; Silva, L.R. Exploring the phenolic profile, antioxidant, antidiabetic and anti-hemolytic potential of Prunus avium vegetal parts. Food Res. Int. 2019, 116, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundao possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef]
- Keating, E.; Calhau, C.; Faria, A.; Martel, F. Interaction of polyphenols with the intestinal and placental absorption of some bioactive compounds. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Academic Press: New York, NY, USA, 2018; pp. 321–336. [Google Scholar] [CrossRef]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 34, 390–406. [Google Scholar] [CrossRef]
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef]
- Tobin, V.; Le Gall, M.; Fioramonti, X.; Stolarczyk, E.; Blazquez, A.G.; Klein, C.; Prigent, M.; Serradas, P.; Cuif, M.H.; Magnan, C.; et al. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes 2008, 57, 555–562. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 2017, 93, 1627–1635. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Goncalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Ataie-Jafari, A.; Hosseini, S.; Karimi, F.; Pajouhi, M. Effects of sour cherry juice on blood glucose and some cardiovascular risk factors improvements in diabetic women: A pilot study. Nutr. Food Sci. 2008, 38, 355–360. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Liu, Y.; Leng, F.; Li, X.; Sun, C.; Chen, K. Bioassay-based isolation and identification of phenolics from sweet cherry that promote active glucose consumption by HepG2 cells. J. Food Sci. 2015, 80, C234–C240. [Google Scholar] [CrossRef]
- Chen, Q.C.; Zhang, W.Y.; Jin, W.; Lee, I.S.; Min, B.S.; Jung, H.J.; Na, M.; Lee, S.; Bae, K. Flavonoids and isoflavonoids from Sophorae Flos improve glucose uptake in vitro. Planta Medica 2010, 76, 79–81. [Google Scholar] [CrossRef]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Noratto, G.D.; Lage, N.N.; Chew, B.P.; Mertens-Talcott, S.U.; Talcott, S.T.; Pedrosa, M.L. Non-anthocyanin phenolics in cherry (Prunus avium L.) modulate IL-6, liver lipids and expression of PPARdelta and LXRs in obese diabetic (db/db) mice. Food Chem. 2018, 266, 405–414. [Google Scholar] [CrossRef]
- Hsu, F.L.; Chen, Y.C.; Cheng, J.T. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Medica 2000, 66, 228–230. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4’-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, Y.; Kwon, O. Selected Phytochemicals and Culinary Plant Extracts Inhibit Fructose Uptake in Caco-2 Cells. Molecules 2015, 20, 17393–17404. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Iwamoto, T.; Okuda, M. Altered functions and expressions of drug transporters in liver, kidney and intestine in disorders of local and remote organs: Possible role of oxidative stress in the pathogenesis. Expert Opin. Drug Metab. Toxicol. 2009, 5, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Monteiro, R.; Pestana, D.; Freitas, V.; Mateus, N.; Azevedo, I.; Calhau, C. Intestinal oxidative state can alter nutrient and drug bioavailability. Oxidative Med. Cell. Longev. 2009, 2, 322–327. [Google Scholar] [CrossRef]
- Roche, M.; Neti, P.V.; Kemp, F.W.; Agrawal, A.; Attanasio, A.; Douard, V.; Muduli, A.; Azzam, E.I.; Norkus, E.; Brimacombe, M.; et al. Radiation-induced reductions in transporter mRNA levels parallel reductions in intestinal sugar transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R173–R182. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]




| Family | Compound | Std * | Concentration (ng/mL of Infusion) |
|---|---|---|---|
| Hydroxycinnamic acids | Chlorogenic acid glucoside | A | 928.9 ± 59.0 |
| cis-Caffeic acid 4-glucoside (cis-Glucocaffeic acid) | B | 56.5 ± 5.3 | |
| cis-3-O-p-Coumaroylquinic acid | A | 15,590.5 ± 1718.7 | |
| trans-Caffeic acid 4-glucoside (trans-Glucocaffeic acid) | B | 58.8 ± 3.9 | |
| 3-O-Caffeoylquinic acid (Chlorogenic acid) | A | 9421.4 ± 1080.0 | |
| beta-D-Glucosyl-2-coumarate (cis-Melilotoside) | C | 511.6 ± 243.2 | |
| Caffeic acid | B | 292.5 ± 44.7 | |
| 1-O-p-Coumaroyl-beta-D-glucose | D | 60.4 ± 7.8 | |
| trans-p-Coumaric acid glucoside | D | 99.1 ± 20.0 | |
| trans-3-O-p-Coumaroylquinic acid | A | 9195.7 ± 398.1 | |
| Ferulic acid glucoside | E | 323.5 ± 32.1 | |
| trans-o-Coumaric acid glucoside | C | 454.3 ± 30.8 | |
| p-Coumaric acid | D | 103.9 ± 7.4 | |
| Ferulic acid | E | 99.3 ± 3.4 | |
| Hydroxybenzoic acids | Gallic acid | F | 1704.5 ± 150.0 |
| Hydroxybenzoic acid glucoside | G | 21.0 ± 1.5 | |
| Salicylic acid | G | 91.7 ± 0.9 | |
| Phenylpropanoic acids | 3-(2-Hydroxyphenyl)propionic acid glucoside | C | 1314.2 ± 112.8 |
| 3-(2-Hydroxyphenyl)propionic acid (Melilotic acid) | C | 556.3 ± 71.8 | |
| Flavanones | Dihydroxymethoxy flavanone-O-pentosylhexoside (I) | H | 144.1 ± 16.9 |
| Tetrahydroxyflavanone-glucoside | H | 406.8 ± 53.5 | |
| Naringenin-7-O-glucoside (Prunin) | H | 1515.9 ± 129.0 | |
| Dihydroxyflavanone-O-pentosylhexoside | H | 112.1 ± 12.1 | |
| Dihydroxymethoxy flavanone-O-pentosylhexoside (II) | H | 90.0 ± 8.5 | |
| Naringenin-O-glucoside | H | 1599.9 ± 136.0 | |
| Dihydrowogonin/Sakuranetin (I) | H | 2326.5 ± 60.8 | |
| Dihydrowogonin-O-glucoside/Sakuranetin-O-glucoside (I) | H | 6620.2 ± 384.2 | |
| Dihydrowogonin/Sakuranetin (II) | H | 5651.5 ± 328.6 | |
| Flavonols | Aromadendrin-O-glucoside | I | 1184.5 ± 108.6 |
| Quercetin-O-rutinoside-O-hexoside | I | 1789.1 ± 219.7 | |
| Kaempferol-O-rutinoside-O-hexoside | I | 2006.8 ± 19.8 | |
| Taxifolin-O-glucoside | I | 1355.3 ± 124.1 | |
| Quercetin-3-O-rutinoside (Rutin) | J | 2200.3 ± 257.0 | |
| Quercetin-3-O-glucoside | I | 560.2 ± 12.0 | |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | I | 3869.3 ± 446.9 | |
| Kaempferol-3-O-glucoside (Astragalin) | I | 773.6 ± 35.1 | |
| Flavones | Tetrahydroxyflavone hexoside (I) | K | 363.1 ± 171.3 |
| Tetrahydroxyflavone hexoside (II) | K | 740.6 ± 349.1 | |
| Chrysin-7-O-glucoside isomer | K | 296.0 ± 142.0 | |
| Chrysin-7-O-glucoside | K | 2357.1 ± 1116.5 | |
| Flavan-3-ols | Catechin | L | 84.5 ± 12.5 |
| Epicatechin | L | 24.3 ± 1.7 | |
| Isoflavones | Genistein-O-glucoside | K | 341.1 ± 160.8 |
| Methyl genistein (Prunetin) | M | 2.4 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto-Peixoto, J.A.; Silva, C.; Costa, A.S.G.; Álvarez-Rivera, G.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P.; Alves, R.C.; Martel, F.; Andrade, N. A Prunus avium L. Infusion Inhibits Sugar Uptake and Counteracts Oxidative Stress-Induced Stimulation of Glucose Uptake by Intestinal Epithelial (Caco-2) Cells. Antioxidants 2024, 13, 59. https://doi.org/10.3390/antiox13010059
Barreto-Peixoto JA, Silva C, Costa ASG, Álvarez-Rivera G, Cifuentes A, Ibáñez E, Oliveira MBPP, Alves RC, Martel F, Andrade N. A Prunus avium L. Infusion Inhibits Sugar Uptake and Counteracts Oxidative Stress-Induced Stimulation of Glucose Uptake by Intestinal Epithelial (Caco-2) Cells. Antioxidants. 2024; 13(1):59. https://doi.org/10.3390/antiox13010059
Chicago/Turabian StyleBarreto-Peixoto, Juliana A., Cláudia Silva, Anabela S. G. Costa, Gerardo Álvarez-Rivera, Alejandro Cifuentes, Elena Ibáñez, M. Beatriz P. P. Oliveira, Rita C. Alves, Fátima Martel, and Nelson Andrade. 2024. "A Prunus avium L. Infusion Inhibits Sugar Uptake and Counteracts Oxidative Stress-Induced Stimulation of Glucose Uptake by Intestinal Epithelial (Caco-2) Cells" Antioxidants 13, no. 1: 59. https://doi.org/10.3390/antiox13010059