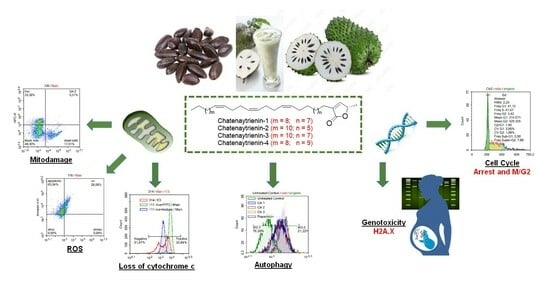
Natural Acetogenins, Chatenaytrienins-1, -2, -3 and -4, Mitochondrial Potential Uncouplers and Autophagy Inducers—Promising Anticancer Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Chemical Materials
2.2. Chemical Synthesis
2.3. Cell Culturing
2.4. Cytotoxicity Assay
2.5. Detection of Mitodamage
2.6. Cell Cycle Analysis
2.7. Assessment of Cytochrome C Loss
2.8. Assessment of Mitochondrial Potential
2.9. Assessment of Autophagy
2.10. Analysis of Genotoxity and Early Apoptosis
2.11. Statistics
3. Results
3.1. Chemistry
3.2. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyoshi, H.; Ohshima, M.; Shimada, H.; Akagi, T.; Iwamura, H.; McLaughlin, J.L. Essential Structural Factors of Annonaceous acetogenins as Potent Inhibitors of Mitochondrial Complex I. Biochim. Biophys. Acta Bioenerg. 1998, 1365, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, U.; Ejaz-ul-Haq, M.; Chudary, Z.; Ahmad, B. Pharmacological Screening of Annona Muricata: A Review. Asian J. Agricul. Biol. 2017, 5, 38–46. [Google Scholar]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A Comprehensive Review on Its Traditional Medicinal Uses, Phytochemicals, Pharmacological Activities, Mechanisms of Action and Toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- Caparros-Lefebvre, D.; Elbaz, A. Possible Relation of Atypical Parkinsonism in the French West Indies with Consumption of Tropical Plants: A Case-Control Study. Lancet 1999, 354, 281–286. [Google Scholar] [CrossRef]
- Caparros-Lefebvre, D.; Steele, J. Atypical parkinsonism on Guadeloupe, comparison with the parkinsonism-dementia complex of Guam, and environmental toxic hypotheses. Environ. Toxicol. Pharmacol. 2005, 19, 407–413. [Google Scholar] [CrossRef]
- Liu, X.X.; Alali, F.Q.; Pilarinou, E.; McLaughlin, J.L. Two Bioactive Mono-Tetrahydrofuran Acetogenins, annoglacins A and B, from Annona glabra. Phytochemistry 1999, 50, 815–821. [Google Scholar] [CrossRef]
- Lannuzel, A.; Michel, P.P.; Caparros-Lefebvre, D.; Abaul, J.; Hocquemiller, R.; Ruberg, M. Toxicity of Annonaceae for Dopaminergic Neurons: Potential Role in Atypical Parkinsonism in Guadeloupe. Mov. Disord. 2002, 17, 84–90. [Google Scholar] [CrossRef]
- Lannuzel, A.; Michel, P.P.; Hoglinger, G.U.; Champy, P.; Jousset, A.; Medja, F.; Lombes, A.; Darios, F.; Gleye, C.; Laurens, A.; et al. The Mitochondrial Complex I Inhibitor Annonacin Is Toxic to Mesencephalic Dopaminergic Neurons by Impairment of Energy Metabolism. Neurosci. 2003, 121, 287–296. [Google Scholar] [CrossRef]
- Champy, P.; Hoglinger, G.U.; Feger, J.; Gleye, C.; Hocquemiller, R.; Laurens, A.; Guerineau, V.; Laprevote, O.; Medja, F.; Lombes, A.; et al. Annonacin, a Lipophilic Inhibitor of Mitochondrial Complex I, Induces Nigral and Striatal Neurodegeneration in Rats: Possible Relevance for Atypical Parkinsonism in Guadeloupe. J. Neurochem. 2004, 88, 63–69. [Google Scholar] [CrossRef]
- Angibaud, G.; Gaultier, C.; Rascol, O. Atypical Parkinsonism and Annonaceae Consumption in New Caledonia. Mov. Disord. 2004, 19, 603–604. [Google Scholar] [CrossRef]
- Potts, L.F.; Luzzio, F.A.; Smith, S.C.; Hetman, M.; Champy, P.; Litvan, I. Annonacin in Asimina Triloba Fruit: Implication for Neurotoxicity. Neurotoxicology 2012, 33, 53–58. [Google Scholar] [CrossRef]
- Tadiparthi, K.; Venkatesh, S. Synthetic Approaches Toward Butenolide-Containing Natural Products. J. Heterocycl. Chem. 2022, 59, 1285–1307. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Bhowmik, A.; Choudhary, P. Muricatacin, a Gateway Molecule to Higher Acetogenin Synthesis. Chem. Asian J. 2020, 15, 3660–3681. [Google Scholar] [CrossRef]
- Kunkalkar, R.A.; Laha, D.; Fernandes, R.A. De novo Protecting-Group-Free Total Synthesis of (+)-Muricadienin, (+)-Ancepsenolide and (+)-3-hexadecyl-5-methylfuran-2(5H)-one. Org. Biomol. Chem. 2016, 14, 9072–9079. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Christian, B.W. Stark Total Synthesis of Muricadienin, the Putative Key Precursor in the Solamin Biosynthesis. Org. Lett. 2014, 16, 5886–5889. [Google Scholar] [CrossRef]
- Yajid, A.I.; Ab Rahman, H.S.; Wong, M.P.K.; Wan Zain, W.Z. Potential benefits of Annona Muricata Incombating Cancer: A Review. Malays. J. Med. Sci. 2018, 25, 5–15. [Google Scholar] [PubMed]
- Naik, A.V.; Sellappan, K. In vitro evaluation of Annona muricata L. (Soursop) Leaf Methanol Extracts on Inhibition of Tumorigenicity and Metastasis of Breast Cancer Cells. Biomarkers 2020, 25, 701–710. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural Compounds with bis-Methylene-Interrupted Z-Double Bonds: Plant Sources, Strategies of Total Synthesis, Biological Activity, and Perspectives. Phytochem. Rev. 2021, 20, 325–342. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Ramazanov, I.R.; Makarova, E.K.; Dzhemilev, U.M. Natural Trienoic Acids as Anticancer Agents: First Stereoselective Synthesis, Cell Cycle Analysis, Induction of Apoptosis, Cell Signaling and Mitochondrial Targeting Studies. Cancers 2021, 13, 1808. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Tuktarova, R.A.; Dzhemilev, U.M. Ti-Catalyzed Cross-Cyclomagnesiation of 1,2-Dienes in the Total Z,Z,Z-Stereoselective Synthesis of Natural Acetogenin–Chatenaytrienin-1. ACS Omega 2019, 4, 14085–14091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’yakonov, V.A.; Tuktarova, R.A.; Ishmukhametova, S.R.; Dzhemilev, U.M. The Facile First Total Synthesis of a Deuterated Analog of Natural Muricadienin. Tetrahedron 2016, 72, 5783–5787. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; D’yakonov, V.A.; Tuktarova, R.A.; Dzhemileva, L.U.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; de Meijere, A. Short Route to the Total Synthesis of Natural Muricadienin, and Investigation of Its Cytotoxic Properties. J. Nat. Prod. 2016, 79, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Dzhemilev, U.M.; D’yakonov, V.A.; Khafizova, L.O.; Ibragimov, A.G. Cyclo- and Carbomagnesiation of 1,2-dienes Catalyzed by Zr Complexes. Tetrahedron 2004, 60, 1287–1291. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Ibragimov, A.G.; Khalilov, L.M.; Dzhemilev, U.M. Novel Mg-Organic Reagents in Organic Synthesis. Cp2TiCl2 Catalyzed Intermolecular Cyclomagnesiation of Cyclic and Acyclic 1,2-Dienes Using Grignard Reagents. Tetrahedron 2008, 64, 10188–10194. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Kh Makarova, E.; Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic Acids and their Topoisomerase I Inhibitory Activity. Chem. Comm. 2013, 49, 8401–8403. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.C.; Brown, C.A. The Reaction of Sodium Borohydride with Nickel Acetate in Ethanol Solution: A Highly Selective Nickel Hydrogenation Catalyst. J. Am. Chem. Soc. 1963, 85, 1005–1006. [Google Scholar] [CrossRef]
- Crabbé, P.; Fillion, H.; André, D.; Luche, J.-L. Efficient Homologation of Acetylenes to Allenes. J. Chem. Soc. Chem. Comm. 1979, 19, 859–860. [Google Scholar] [CrossRef]
- Brandänge, S.; Flodman, L.; Norberg, A. Studies on the Intramolecular Claisen Condensation: Facile Synthesis of Tetronic Acids. J. Org. Chem. 1984, 49, 927–928. [Google Scholar] [CrossRef]
- Spence, J.T.J.; George, J.H. Biomimetic Total Synthesis of ent-Penilactone A and Penilactone B. Org. Lett. 2013, 15, 3891–3893. [Google Scholar] [CrossRef]
- Daddiouaissa, D.; Amid, A. Anticancer Activity of Acetogenins from Annona Muricata Fruit. IIUM Med. J. Malays. 2018, 17, 103–112. [Google Scholar] [CrossRef]
- Gleye, C.; Raynaud, S.; Hocquemiller, R.; Laurens, A.; Fourneau, C.; Serani, L.; Laprévote, O.; Roblot, F.; Leboeuf, M.; Fournet, A.; et al. Muricadienin, Muridienins and Chatenaytrienins, the Early Precursors of Annonaceous acetogenins. Phytochemistry 1998, 47, 749–754. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural Substances (Acetogenins) from the Family Annonaceae are Powerful Inhibitors of Mitochondrial NADH Dehydrogenase (Complex I). Biochem. J. 1994, 301, 161–167. [Google Scholar] [CrossRef] [Green Version]
- La Forge, F.B.; Haller, H.L.; Smith, L.E. The Determination of the Structure of Rotenone. Chem. Rev. 1933, 12, 181–213. [Google Scholar] [CrossRef]
- Ikezawa, N.; Ifuku, K.; Endo, T.; Sato, F. Inhibition of Photosystem II of Spinach by the Respiration Inhibitors Piericidin A and Thenoyltrifluoroacetone. Biosci. Biotech. Biochem. 2002, 66, 1925–1929. [Google Scholar] [CrossRef] [Green Version]
- Masaya, N.; Takashi, I.; Shinichi, A.; Youichi, H.; Hiroshi, M.; Rika, S.; Kuniaki, T. Synthetic Studies on Oligomycins. Synthesis of The Oligomycin B Spiroketal and Polypropionate Portions. Bull. Chem. Soc. Jap. 1995, 68, 967–989. [Google Scholar]
- Stewart, M.J.; Steenkamp, V. The Biochemistry and Toxicity of Atractyloside: A Review. Ther. Drug Monit. 2000, 22, 641–649. [Google Scholar] [CrossRef]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-Dinitrophenol (DNP): A Weight Loss Agent with Significant Acute Toxicity and Risk of Death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Heytler, P.G.; Prichard, W.W. A New Class of Uncoupling Agents—Carbonyl Cyanide Phenylhydrazones. Biochem. Biophys. Res. Comm. 1962, 7, 272–275. [Google Scholar] [CrossRef]
- Rose, L.; Jenkins, A.T. A The Effect of the Ionophore Valinomycin on Biomimetic Solid Supported Lipid DPPTE/EPC Membranes. Bioelectrochemistry 2007, 70, 387–393. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican Medicinal Plants Used for Cancer Treatment: Pharmacological, Phytochemical and Ethnobotanical Studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef]
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative Activity of Aqueous Leaf Extract of Annona Muricata L. on the Prostate, BPH-1 Cells, and Some Target Genes. Integr. Cancer Ther. 2015, 14, 65–74. [Google Scholar] [CrossRef]
- Pieme, C.A.; Kumar, S.G.; Dongmo, M.S.; Moukette, B.M.; Boyoum, F.F.; Ngogang, J.Y.; Saxena, A.K. Antiproliferative Activity and Induction of Apoptosis by Annona Muricata (Annonaceae) Extract on Human Cancer Cells. BMC Compl. Altern. Med. 2014, 14, 516–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasasti, E.; Rachmani, N.; Suhesti, T.S.; Widiastuti, R. The Breast of Anticancer from Leaf Extract of Annona Muricata Against Cell Line in T47D. Int. J. Appl. Sci. Techn. 2012, 2, 157–164. [Google Scholar]
- Sulistyoningrum, E.; Prasasti, E.; Rachmani, N.; Baroroh, H.N.; Rujito, L. Annona muricata Leaves Extract Reduce Proliferative Indexes and Improve Histological Changes in Rat’s Breast Cancer. J. Appl. Pharm. Sci. 2017, 7, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Quispe, A.; Zavala, D.; Rojas, J.; Posso, M.; Vaisberg, A. Efecto Citotóxico Selectivo In Vitro De Muricin H (Acetogenina de Annona Muricata) en Cultivos Celulares de Cáncer de Pulmón. Rev. Peru. Med. Exp. Sal. Públ. 2006, 23, 265–269. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6, 469–471. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Sylvester, P.W. Optimization of the Tetrazolium Dye (MTT) Colorimetric Assay for Cellular Growth and Viability. Methods Mol. Biol. 2011, 716, 157–168. [Google Scholar] [PubMed]
- Hofsteen, P.; Karassina, N.; Cali, J.J.; Vidugiriene, J. A Luminescence Assay to Quantify Cell Viability in Real Time. Methods Mol. Biol. 2021, 2255, 187–196. [Google Scholar]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar]
- Ashrafi, G.; Schwarz, T.L. The Pathways of Mitophagy for Quality Control and Clearance of Mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, E. The Mitochondrion: Is it Central to Apoptosis? Science 2001, 292, 624–626. [Google Scholar] [CrossRef]
- Krysko, D.V.; Roels, F.; Leybaert, L.; D’Herde, K. Mitochondrial Transmembrane Potential Changes Support the Concept of Mitochondrial Heterogeneity During Apoptosis. J. Histochem. Cytochem. 2001, 49, 1277–1284. [Google Scholar] [CrossRef] [Green Version]
- Gollapudi, S.; McCormick, M.J.; Gupta, S. Changes in Mitochondrial Membrane Potential and Mitochondrial Mass Occur Independent of the Activation of Caspase-8 and Caspase-3 During CD95-Mediated Apoptosis in Peripheral Blood T Cells. Int. J. Oncol. 2003, 22, 597–600. [Google Scholar] [PubMed]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and Function in Cell Death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome C Release from Mitochondria. Cell Death Diff. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Krippner, A.; Matsuno-Yagi, A.; Gottlieb, R.A.; Babior, B.M. Loss of Function of Cytochrome c in Jurkat Cells Undergoing Fas-mediated Apoptosis. J. Biol. Chem. 1996, 271, 21629–21636. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.J.; Brand, M.D. Reactive Oxygen Species Production by Mitochondria. Methods Mol. Biol. 2009, 554, 165–181. [Google Scholar]
- Cadenas, E.; Davies, K.J. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free Radical Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, Oncosis, and Necrosis. An Overview of Cell Death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived Red Fluorescence is Not a Reliable Indicator of Intracellular Superoxide Formation: Another Inconvenient Truth. Free Rad. Biol. Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.M.; Adameová, A.; Barile, L.; Cabrera-Fuentes, H.A.; Lazou, A.; Pagliaro, P.; Stensløkken, K.-O.; Garcia-Dorado, D. Mitochondrial and Mitochondrial-Independent Pathways of Myocardial Cell Death During Ischaemia and Reperfusion Injury. J. Cell. Mol. Med. 2020, 24, 3795–3806. [Google Scholar] [CrossRef] [Green Version]
- Angwa, L.M.; Jiang, Y.; Pei, J.; Sun, D. Antioxidant Phytochemicals for the Prevention of Fluoride-Induced Oxidative Stress and Apoptosis: A Review. Biol. Trace Elem. Res. 2022, 200, 1418–1441. [Google Scholar] [CrossRef]
- Dong, D.; Wu, J.; Sheng, L.; Gong, X.; Zhang, Z.; Yu, C. FUNDC1 Induces Apoptosis and Autophagy under Oxidative Stress Via PI3K/Akt/Mtor Pathway in Cataract Lens Cells. Curr. Eye Res. 2022, 47, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Jia, F.Y.; Chen, X.; Wang, Z.H.; Jin, W.Y.; Yang, J. Salidroside Alleviates Oxidative Stress and Apoptosis Via AMPK/Nrf2 Pathway in DHT-induced Human Granulosa Cell Line KGN. Arch. Biochem. Biophys. 2022, 715, 109094. [Google Scholar] [CrossRef]
- Spindler, K.D.; Spindler-Barth, M.; Londershausen, M. Chitin Metabolism: A Target for Drugs Against Parasites. Parasitol. Res. 1990, 76, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Biswas, S.; Pal, K.; Ray, D.P. Annona squamosa as a Potential Botanical Insecticide for Agricultural Domains: A Review. Int. J. Biores. Sci. 2018, 5, 81–89. [Google Scholar] [CrossRef]
- Nava-Tapia, D.A.; Cayetano-Salazar, L.; Herrera-Zúñiga, L.D.; Bello-Martínez, J.; Mendoza-Catalán, M.A.; Navarro-Tito, N. Brazilin: Biological Activities and Therapeutic Potential in Chronic Degenerative Diseases and Cancer. Pharmacol. Res. 2022, 175, 106023. [Google Scholar] [CrossRef]
- Ohsumi, Y. Historical Landmarks of Autophagy Research. Cell Res. Vol. 2014, 24, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbańska, K.; Orzechowski, A. The Secrets of Alternative Autophagy. Cells 2021, 10, 3241. [Google Scholar] [CrossRef]
- Codogno, P.; Mehrpour, M.; Proikas-Cezanne, T. Canonical and Non-Canonical Autophagy: Variations on a Common Theme of Self-Eating? Nat. Rev. Molec. Cell Biol. 2012, 13, 7–12. [Google Scholar] [CrossRef]
- Jesus, T.T.; Oliveira, P.F.; Sousa, M.; Cheng, C.Y.; Alves, M.G. Mammalian Target of Rapamycin (Mtor): A Central Regulator of Male Fertility? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 235–253. [Google Scholar] [CrossRef]
- Castets, P.; Lin, S.; Rion, N.; Di Fulvio, S.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Rüegg, M.A. Sustained Activation of mTORC1 in Skeletal Muscle Inhibits Constitutive and Starvation-Induced Autophagy and Causes a Severe, Late-Onset Myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731–655740. [Google Scholar] [CrossRef]
- Lannuzel, A.; Höglinger, G.U.; Verhaeghe, S.; Gire, L.; Belson, S.; Escobar-Khondiker, M.; Poullain, P.; Oertel, W.H.; Hirsch, E.C.; Dubois, B.; et al. Atypical Parkinsonism in Guadeloupe: A Common Risk Factor for Two Closely Related Phenotypes? Brain 2007, 130, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Mutakin, M.; Fauziati, R.; Fadhilah, F.N.; Zuhrotun, A.; Amalia, R.; Hadisaputri, Y.E. Pharmacological activities of Soursop (Annona muricata Lin.). Molecules 2022, 27, 1201. [Google Scholar] [CrossRef]
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M.A. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Compl. Altern. Med. 2016, 16, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Herrera, N.; Pérez-Plasencia, C.; Castro-Torres, V.A.; Martínez-Vázquez, M.; González-Esquinca, A.R.; Zentella-Dehesa, A. Selective Acetogenins and Their Potential as Anticancer Agents. Front. Pharmacol. 2019, 10, 783–795. [Google Scholar] [CrossRef]
- Qian, J.Q.; Sun, P.; Pan, Z.Y.; Fang, Z.Z. Annonaceous acetogenins Reverses Drug Resistance of Human Hepatocellular Carcinoma BEL-7402/5-FU and HepG2/ADM Cell Lines. Int. J. Clin. Exp. Pathol. 2015, 8, 11934–11944. [Google Scholar]
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A Novel Promising Natural-Derived Drug That Inhibits Tumorigenicity and Metastasis of Pancreatic Cancer Cells in Vitro And In Vivo Through Altering Cell Metabolism. Cancer Lett. 2012, 323, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.S.; Chang, H.L.; Chen, H.W.; Kuo, F.C.; Liaw, C.C.; Su, J.H.; Wu, Y.C. Selective Cytotoxicity of Squamocin on T24 Bladder Cancer Cells at the S-Phase Via A Bax-, Bad- And Caspase-3-Related Pathways. Life Sci. 2006, 78, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Moscoso, N.; Romero Benavides, J.C.; Ramirez Orellana, M.I.; Ojeda, K.; Granda, G.; Ratoviski, E.A.; Ostrosky-Wegman, P. Cytotoxic and Genotoxic Effects of Extracts from Annona montana M. fruit. Food Agricul. Immunol. 2016, 27, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Le Ven, J.; Schmitz-Afonso, I.; Lewin, G.; Brunelle, A.; Touboul, D.; Champy, P. Identification of the Environmental Neurotoxins Annonaceous acetogenins in an Annona cherimolia Mill. Alcoholic Beverage Using HPLC-ESI-LTQ-Orbitrap. J. Agricul. Food Chem. 2014, 62, 8696–8704. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential Therapeutic Applications of The Genus Annona: Local and Traditional Uses and Pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef]
- Amala Dev, A.R.; Joseph, S.M. Anticancer potential of Annona genus: A detailed review. J. Ind. Chem. Soc. 2021, 98, 100231–100246. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Sivaganesh, V.; Sivaganesh, V.; Scanlon, C.; Iskander, A.; Maher, S.; Lê, T.; Peethambaran, B. Protein Tyrosine Phosphatases: Mechanisms in Cancer. Int. J. Mol. Sci. 2021, 22, 12865. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX Phosphorylation: A Marker for DNA Damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar]
- Kuo, L.J.; Yang, L.X. γ-H2AX—A Novel Biomarker for DNA Double-strand Breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139*. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Yaffe, M.B. Protein Regulation in Signal Transduction. Cold Spring Harb. Perspect. Biol. 2016, 8, a005918. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.J.; McCormick, F. The molecular pathology of cancer. Nat. Rev. Clin. Oncol. 2010, 7, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and Clinical Significance of Fibroblast Growth Factor 2 (FGF2 /bFGF) in Malignancies of Solid and Hematological Cancers for Personalized Therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar] [CrossRef] [Green Version]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK Signaling Pathways Inhibitors as Anticancer Agents: Structural and Pharmacological Perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef]
- Schunter, A.J.; Yue, X.; Hummon, A.B. Phosphoproteomics of Colon Cancer Metastasis: Comparative Mass Spectrometric Analysis of the Isogenic Primary and Metastatic Cell Lines SW480 and SW620. Analyt. Bioanal. Chem. 2017, 409, 1749–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweppe, D.K.; Rigas, J.R.; Gerber, S.A. Quantitative Phosphoproteomic Profiling of Human Non-Small Cell Lung Cancer Tumors. J. Proteom. 2013, 91, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Zwick, E.; Bange, J.; Ullrich, A. Receptor Tyrosine Kinase Signalling as a Target for Cancer Intervention Strategies. Endocr.-Rel. Cancer 2001, 8, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.S.; Xu, P.Z.; Gottlob, K.; Chen, M.L.; Sokol, K.; Shiyanova, T.; Roninson, I.; Weng, W.; Suzuki, R.; Tobe, K.; et al. Growth Retardation and Increased Apoptosis in Mice with Homozygous Disruption of the Akt1 Gene. Gen. Dev. 2001, 15, 2203–2208. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.R.; Toker, A. Akt Isoform-specific Signaling in Breast Cancer: Uncovering an Anti-Migratory Role for Paladin. Cell Adhes. Migr. 2011, 5, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, M.; Pal, R.; Nelvagal, H.R.; Lotfi, P.; Stinnett, G.R.; Seymour, M.L.; Chaudhury, A.; Bajaj, L.; Bondar, V.V.; Bremner, L.; et al. mTORC1-independent TFEB Activation Via Akt Inhibition Promotes Cellular Clearance in Neurodegenerative Storage Diseases. Nature Comm. 2017, 8, 14338–14357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momand, J.; Jung, D.; Wilczynski, S.; Niland, J. The MDM2 Gene Amplification Database. Nucl. Acid. Res. 1998, 26, 3453–3459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the P53-MDM2 Pathway for Neuroblastoma Therapy: Rays of Hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 Pathways in DNA Damage Signaling and Cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Chapter 2—Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Stud. Nat. Prod. Chem. 2017, 54, 21–86. [Google Scholar]
- Muslimovic, A.; Ismail, I.H.; Gao, Y.; Hammarsten, O. An Optimized Method for Measurement of Gamma-H2AX in Blood Mononuclear and Cultured Cells. Nat. Prot. 2008, 3, 1187–1193. [Google Scholar] [CrossRef]
- Tanaka, T.; Huang, X.; Dorota Halicka, H.; Zhao, H.; Traganos, F.; Albino, A.P.; Dai, W.; Darzynkiewicz, Z. Cytometry of ATM Activation and Histone H2AX Phosphorylation to Estimate Extent of DNA Damage Induced by Exogenous Agents. Cytometry 2007, 71, 648–661. [Google Scholar] [CrossRef] [Green Version]
- Wan, R.; Mo, Y.; Tong, R.; Gao, M.; Zhang, Q. Determination of Phosphorylated Histone H2AX in Nanoparticle-Induced Genotoxic Studies. Methods Mol. Biol. 2019, 1894, 145–159. [Google Scholar]
- Ewald, B.; Sampath, D.; Plunkett, W. H2AX Phosphorylation Marks Gemcitabine-Induced Stalled Replication Forks and Their Collapse Upon S-phase Checkpoint. Mol. Cancer Ther. 2007, 6, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Plappert-Helbig, U.; Libertini, S.; Frieauff, W.; Theil, D.; Martus, H.J. Gamma-H2AX Immunofluorescence for the Detection of Tissue-Specific Genotoxicity In Vivo. Env. Mol. Mutagen. 2019, 60, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, R.; Lu, N.; Wang, J.; Yin, Y.; Ding, Y.; Zhang, X.; Gui, H.; Sun, Q.; Duan, H.; Zhang, L.; et al. An Oxidative Analogue of Gambogic Acid-Induced Apoptosis of Human Hepatocellular Carcinoma Cell Line Hepg2 Is Involved in its Anticancer Activity In Vitro. Eur. J. Cancer Prev. 2010, 19, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.B.; Schuler, M.; Echeverri, F.; Green, D.R. Caspase-3 is the Primary Activator of Apoptotic DNA Fragmentation Via DNA Fragmentation Factor-45/Inhibitor of Caspase-Activated Dnase Inactivation. J. Biol. Chem. 1999, 274, 30651–30656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase Family Proteases and Apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner Caspase-3, -6, and -7 Perform Distinct, Non-Redundant Roles During the Demolition Phase of Apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shu, Y.; Zhang, Q.; Liu, B.; Xia, J.; Qiu, M.; Miao, H.; Li, M.; Zhu, R. Dihydromyricetin Induces Apoptosis and Inhibits Proliferation in Hepatocellular Carcinoma Cells. Oncol. Lett. 2014, 8, 1645–1651. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, K.; Chen, G.; Lindsten, T.; White, E. BAX and BAK Mediate p53-independent Suppression of Tumorigenesis. Cancer Cell 2002, 2, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, C.; Nöldner, M.; Abdel-Kader, R.; Johnson-Anuna, L.N.; Gibson Wood, W.; Müller, W.E.; Eckert, G.P. Bcl-2 upregulation and Neuroprotection in Guinea Pig Brain Following Chronic Simvastatin Treatment. Neurobiol. Dis. 2007, 25, 438–445. [Google Scholar] [CrossRef]












| Jurkat | K562 | U937 | HL60 | Hek293 | Fibroblasts (PCS-201-018) | |
|---|---|---|---|---|---|---|
| 1 | 0.09 ± 0.02 (0.08–0.99) | 0.10 ± 0.03 (0.09-0.11) | 0.09 ± 0.02 (0.08–0.11) | 0.15 ± 0.01 (0.14–0.16) | 0.53 ± 0.02 (0.51–0.54) | 0.69 ± 0.04 (0.67–0.81) |
| 2 | 0.12 ± 0.06 (0.11–0.13) | 0.18 ± 0.05 (0.17–0.19) | 0.12 ± 0.01 (0.11–0.13) | 0.13 ± 0.02 (0.12–0.14) | 0.63 ± 0.03 (0.61–0.64) | 0.79 ± 0.02 (0.77–0.82) |
| 3 | 0.15 ± 0.04 (0.14–0.16) | 0.09 ± 0.01 (0.08–0.1) | 0.18 ± 0.02 (0.17–0.19) | 0.14 ± 0.04 (0.13–0.15) | 0.78 ± 0.04 (0.76–0.79) | 0.83 ± 0.05 (0.81–0.84) |
| 4 | 0.16 ± 0.01 (0.15–0.17) | 0.13 ± 0.04 (0.12–0.14) | 0.13 ± 0.05 (0.12–0.14) | 0.19 ± 0.02 (0.18–0.21) | 0.53 ± 0.01 (0.52–0.54) | 0.98 ± 0.04 (0.96–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhemileva, L.U.; Tuktarova, R.A.; Dzhemilev, U.M.; D’yakonov, V.A. Natural Acetogenins, Chatenaytrienins-1, -2, -3 and -4, Mitochondrial Potential Uncouplers and Autophagy Inducers—Promising Anticancer Agents. Antioxidants 2023, 12, 1528. https://doi.org/10.3390/antiox12081528
Dzhemileva LU, Tuktarova RA, Dzhemilev UM, D’yakonov VA. Natural Acetogenins, Chatenaytrienins-1, -2, -3 and -4, Mitochondrial Potential Uncouplers and Autophagy Inducers—Promising Anticancer Agents. Antioxidants. 2023; 12(8):1528. https://doi.org/10.3390/antiox12081528
Chicago/Turabian StyleDzhemileva, Lilya U., Regina A. Tuktarova, Usein M. Dzhemilev, and Vladimir A. D’yakonov. 2023. "Natural Acetogenins, Chatenaytrienins-1, -2, -3 and -4, Mitochondrial Potential Uncouplers and Autophagy Inducers—Promising Anticancer Agents" Antioxidants 12, no. 8: 1528. https://doi.org/10.3390/antiox12081528