n-3 Polyunsaturated Fatty Acid Supplementation Affects Oxidative Stress Marker Levels in Patients with Type II Intestinal Failure: A Randomized Double Blind Trial
Abstract
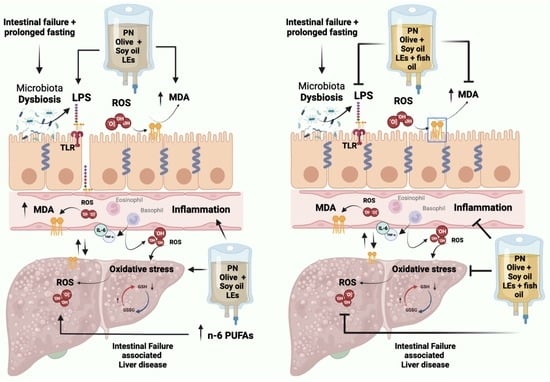
:1. Introduction
2. Materials and Methods
2.1. Study Design, Ethics Approval, and Interventions
2.2. Anthropometry
2.3. Evaluation of Biochemical Parameters
2.3.1. Evaluation of Oxidative and Antioxidant Biomarkers
2.3.2. Determination of LPS and Other Biochemical Parameters
2.4. Evaluation of Clinical Parameters
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuerda, C.; Pironi, L.; Arends, J.; Bozzetti, F.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin. Nutr. 2021, 40, 5196–5220. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Corcos, O.; Forbes, A.; Holst, M.; Joly, F.; Jonkers, C.; Klek, S.; Lal, S.; Blaser, A.R.; Rollins, K.E.; et al. Intestinal failure in adults: Recommendations from the ESPEN expert groups. Clin. Nutr. 2018, 37, 1798–1809. [Google Scholar] [CrossRef] [Green Version]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [Green Version]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef]
- Thibault, R.; Abbasoglu, O.; Ioannou, E.; Meija, L.; Ottens-Oussoren, K.; Pichard, C.; Rothenberg, E.; Rubin, D.; Siljamäki-Ojansuu, U.; Vaillant, M.-F.; et al. ESPEN guideline on hospital nutrition. Clin. Nutr. 2021, 40, 5684–5709. [Google Scholar] [CrossRef]
- Mirtallo, J.M.; Dasta, J.F.; Kleinschmidt, K.C.; Varon, J. State of the Art Review: Intravenous Fat Emulsions: Current Applications, Safety Profile, and Clinical Implications. Ann. Pharmacother. 2010, 44, 688–700. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Sadu Singh, B.K.; Narayanan, S.S.; Khor, B.H.; Sahathevan, S.; Abdul Gafor, A.H.; Fiaccadori, E.; Sundram, K.; Karupaiah, T. Composition and Functionality of Lipid Emulsions in Parenteral Nutrition: Examining Evidence in Clinical Applications. Front. Pharmacol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Allan, P.; Lal, S. Intestinal failure: A review [version 1; peer review: 2 approved]. F1000Research 2018, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Pironi, L.; Sasdelli, A.S. Intestinal Failure-Associated Liver Disease. Clin. Liver Dis. 2019, 23, 279–291. [Google Scholar] [CrossRef]
- Zugasti Murillo, A.; Petrina Jáuregui, E.; Elizondo Armendáriz, J. Hepatopatía asociada a nutrición parenteral y emulsiones lipídicas. Endocrinol. Nutr. 2015, 62, 285–289. [Google Scholar] [CrossRef]
- Honeywell, S.; Zelig, R.; Rigassio Radler, D. Impact of Intravenous Lipid Emulsions Containing Fish Oil on Clinical Outcomes in Critically Ill Surgical Patients: A Literature Review. Nutr. Clin. Pract. 2019, 34, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundi, M.S.; Bonnes, S.L.; Salonen, B.R.; McMahon, M.M.; Martindale, R.; Hurt, R.T. Clinical application of fish-oil intravenous lipid emulsion in adult home parenteral nutrition patients. Nutr. Clin. Pract. 2021, 36, 839–852. [Google Scholar] [CrossRef]
- Gong, Q.; Zhu, P.; Zhang, B.; Shu, C.; Ding, Z.; Wu, J.; Zhang, B.; Chen, X.-P. Safety and efficacy of n-3 fatty acid-based parenteral nutrition in patients with obstructive jaundice: A propensity-matched study. Eur. J. Clin. Nutr. 2018, 72, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Lou, P.-H.; Lucchinetti, E.; Wawrzyniak, P.; Morsy, Y.; Wawrzyniak, M.; Scharl, M.; Krämer, S.D.; Rogler, G.; Hersberger, M.; Zaugg, M. Choice of Lipid Emulsion Determines Inflammation of the Gut-Liver Axis, Incretin Profile, and Insulin Signaling in a Murine Model of Total Parenteral Nutrition. Mol. Nutr. Food Res. 2021, 65, 2000412. [Google Scholar] [CrossRef]
- Lee, W.S.; Chew, K.S.; Ng, R.T.; Kasmi, K.E.; Sokol, R.J. Intestinal failure-associated liver disease (IFALD): Insights into pathogenesis and advances in management. Hepatol. Int. 2020, 14, 305–316. [Google Scholar] [CrossRef]
- Watanabe, Y.; Miyoshi, N.; Fujino, S.; Takahashi, H.; Haraguchi, N.; Hata, T.; Matsuda, C.; Yamamoto, H.; Doki, Y.; Mori, M.; et al. Cumulative Inflammation Could Be a Risk Factor for Intestinal Failure in Crohn’s Disease. Dig. Dis. Sci. 2019, 64, 2280–2285. [Google Scholar] [CrossRef]
- Osowska, S.; Kunecki, M.; Sobocki, J.; Tokarczyk, J.; Majewska, K.; Omidi, M.; Radkowski, M.; Fisk, H.L.; Calder, P.C. Effect of changing the lipid component of home parenteral nutrition in adults. Clin. Nutr. 2019, 38, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Galicia-Moreno, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Álvarez-Torres, T.; Falcón, D.; Higuera-de la Tijera, F.; Béjar, Y.L.; Cordero-Pérez, P.; Muñoz-Espinosa, L.; Pérez-Hernández, J.L.; et al. Behavior of Oxidative Stress Markers in Alcoholic Liver Cirrhosis Patients. Oxid. Med. Cell. Longev. 2016, 2016, 9370565. [Google Scholar] [CrossRef] [Green Version]
- Langness, S.; Kojima, M.; Coimbra, R.; Eliceiri, B.P.; Costantini, T.W. Enteric glia cells are critical to limiting the intestinal inflammatory response after injury. Am. J. Physiol. -Gastrointest. Liver Physiol. 2017, 312, G274–G282. [Google Scholar] [CrossRef] [Green Version]
- Khanum, R.; Thevanayagam, H. Lipid peroxidation: Its effects on the formulation and use of pharmaceutical emulsions. Asian J. Pharm. Sci. 2017, 12, 401–411. [Google Scholar] [CrossRef]
- Steger, P.J.K.; Mühlebach, S.F. Lipid Peroxidation of Intravenous Lipid Emulsions and All-in-One Admixtures in Total Parenteral Nutrition Bags: The Influence of Trace Elements. J. Parenter. Enter. Nutr. 2000, 24, 37–41. [Google Scholar] [CrossRef]
- Fallon, E.M.; Le, H.D.; Puder, M. Prevention of parenteral nutrition-associated liver disease: Role of ω-3 fish oil. Curr. Opin. Organ Transplant. 2010, 15, 334–340. [Google Scholar] [CrossRef]
- Kirk, C.; Haigh, L.; Thompson, N.P.; Pearce, M.; Jones, D.E.; Mathers, J.C. The effects of different parenteral nutrition lipid formulations on clinical and laboratory endpoints in patients receiving home parenteral nutrition: A systematic review. Clin. Nutr. 2022, 41, 80–90. [Google Scholar] [CrossRef]
- Klek, S. Omega-3 Fatty Acids in Modern Parenteral Nutrition: A Review of the Current Evidence. J. Clin. Med. 2016, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Intravenous Lipid Emulsions to Deliver Bioactive Omega-3 Fatty Acids for Improved Patient Outcomes. Mar. Drugs 2019, 17, 274. [Google Scholar] [CrossRef] [Green Version]
- Ottestad, I.; Nordvi, B.; Vogt, G.; Holck, M.; Halvorsen, B.; Brønner, K.W.; Retterstøl, K.; Holven, K.B.; Nilsson, A.; Ulven, S.M. Bioavailability of n-3 fatty acids from n-3-enriched foods and fish oil with different oxidative quality in healthy human subjects: A randomised single-meal cross-over study. J. Nutr. Sci. 2016, 5, e43. [Google Scholar] [CrossRef] [Green Version]
- Pironi, L.; Agostini, F.; Guidetti, M. Intravenous lipids in home parenteral nutrition. World Rev. Nutr. Diet 2015, 112, 141–149. [Google Scholar] [CrossRef]
- Klek, S.; Mankowska-Wierzbicka, D.; Scislo, L.; Walewska, E.; Pietka, M.; Szczepanek, K. High Dose Intravenous Fish Oil Reduces Inflammation-A Retrospective Tale from Two Centers. Nutrients 2020, 12, 2865. [Google Scholar] [CrossRef]
- Pradelli, L.; Mayer, K.; Klek, S.; Omar Alsaleh, A.J.; Clark, R.A.C.; Rosenthal, M.D.; Heller, A.R.; Muscaritoli, M. ω-3 Fatty-Acid Enriched Parenteral Nutrition in Hospitalized Patients: Systematic Review With Meta-Analysis and Trial Sequential Analysis. J. Parenter. Enter. Nutr. 2020, 44, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Heller, A.R.; Rössler, S.; Litz, R.J.; Stehr, S.N.; Heller, S.C.; Koch, R.; Koch, T. Omega-3 fatty acids improve the diagnosis-related clinical outcome*. Crit. Care Med. 2006, 34, 972–979. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Mirtallo, J.; Canada, T.; Johnson, D.; Kumpf, V.; Petersen, C.; Sacks, G.; Seres, D.; Guenter, P. Safe Practices for Parenteral Nutrition. J. Parenter. Enter. Nutr. 2004, 28, S39–S70. [Google Scholar] [CrossRef]
- Pironi, L.; Guidetti, M.; Verrastro, O.; Iacona, C.; Agostini, F.; Pazzeschi, C.; Sasdelli, A.S.; Melchiorre, M.; Ferreri, C. Functional lipidomics in patients on home parenteral nutrition: Effect of lipid emulsions. World J. Gastroenterol. 2017, 23, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.-C.; Chaudière, J. Reactions of 1-Methyl-2-phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Manuel-y-Keenoy, B.; Nonneman, L.; De Bosscher, H.; Vertommen, J.; Schrans, S.; Klütsch, K.; De Leeuw, I. Effects of intravenous supplementation with α-tocopherol in patients receiving total parenteral nutrition containing medium- and long-chain triglycerides. Eur. J. Clin. Nutr. 2002, 56, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Davidson, M.H.; Maki, K.C.; Bays, H.; Carter, R.; Ballantyne, C.M. Effects of prescription omega-3-acid ethyl esters on lipoprotein particle concentrations, apolipoproteins AI and CIII, and lipoprotein-associated phospholipase A<sub>2</sub> mass in statin-treated subjects with hypertriglyceridemia. J. Clin. Lipidol. 2009, 3, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef] [Green Version]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Roberts-Lee, K.; Borcea, C.; Smith, H.M.; Midgette, Y.; Shah, R. Omega-3 polyunsaturated fatty acids attenuate inflammatory activation and alter differentiation in human adipocytes. J. Nutr. Biochem. 2019, 64, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kosek, V.; Heczkova, M.; Novak, F.; Meisnerova, E.; Novákova, O.; Zelenka, J.; Bechynska, K.; Vrzacova, N.; Suttnar, J.; Hlavackova, A.; et al. The ω-3 Polyunsaturated Fatty Acids and Oxidative Stress in Long-Term Parenteral Nutrition Dependent Adult Patients: Functional Lipidomics Approach. Nutrients 2020, 12, 2351. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Vagianos, C.E.; Charonis, A.; Nikolopoulou, V.N.; Scopa, C.D. Intestinal failure in obstructive jaundice. World J. Gastroenterol. 2005, 11, 3806–3807. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, J.; Osowska, S.; Kunecki, M.; Sobocki, J.; Ładyżyński, P.; Giebułtowicz, J. Antioxidant balance in plasma of patients on home parenteral nutrition: A pilot study comparing three different lipid emulsions. Clin. Nutr. 2021, 40, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Flota, X.; Castillo-Martínez, L.; Reyes-Ramírez, A.L.; Osorio-Alamillo, Y.; Murguía-Vázquez, M.; Serralde-Zúñiga, A.E. Short article: Frequency, pathophysiology, and clinical classification of intestinal failure type II and III at a tertiary referral center. Eur. J. Gastroenterol. Hepatol. 2019, 31, 123–127. [Google Scholar] [CrossRef]
- LAL, S.; Teubner, A.; Shaffer, J.L. Review article: Intestinal failure. Aliment. Pharmacol. Ther. 2006, 24, 19–31. [Google Scholar] [CrossRef]
- Ławiński, M.; Singer, P.; Gradowski, Ł.; Gradowska, A.; Bzikowska, A.; Majewska, K. Predicted versus measured resting energy expenditure in patients requiring home parenteral nutrition. Nutrition 2015, 31, 1328–1332. [Google Scholar] [CrossRef]
- Skallerup, A.; Nygaard, L.; Olesen, S.S.; Vinter-Jensen, L.; Køhler, M.; Rasmussen, H.H. Can We Rely on Predicted Basal Metabolic Rate in Patients With Intestinal Failure on Home Parenteral Nutrition? J. Parenter. Enter. Nutr. 2017, 41, 1139–1145. [Google Scholar] [CrossRef]
- Antebi, H.; Mansoor, O.; Ferrier, C.; Tetegan, M.; Morvan, C.; Rangaraj, J.; Alcindor, L. Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: Comparison of 2 fat emulsions. J. Parenter. Enter. Nutr. 2004, 28, 142–148. [Google Scholar] [CrossRef]
- Donoghue, V.; Schleicher, G.K.; Spruyt, M.G.L.; Malan, L.; Nel, D.G.; Calder, P.C.; Blaauw, R. Four-oil intravenous lipid emulsion effect on plasma fatty acid composition, inflammatory markers and clinical outcomes in acutely ill patients: A randomised control trial (Foil fact). Clin. Nutr. 2019, 38, 2583–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klek, S.; Chambrier, C.; Singer, P.; Rubin, M.; Bowling, T.; Staun, M.; Joly, F.; Rasmussen, H.; Strauss, B.J.; Wanten, G.; et al. Four-week parenteral nutrition using a third generation lipid emulsion (SMOFlipid)—A double-blind, randomised, multicentre study in adults. Clin. Nutr. 2013, 32, 224–231. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.O.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, T.; Zhao, S.; Li, W.; Ma, L.; Ding, M.; Liu, Y. Effects of n-3 polyunsaturated fatty acids high fat diet intervention on the synthesis of hepatic high-density lipoprotein cholesterol in obesity-insulin resistance rats. Lipids Health Dis. 2016, 15, 81. [Google Scholar] [CrossRef] [Green Version]
- Pizzini, A.; Lunger, L.; Demetz, E.; Hilbe, R.; Weiss, G.; Ebenbichler, C.; Tancevski, I. The Role of Omega-3 Fatty Acids in Reverse Cholesterol Transport: A Review. Nutrients 2017, 9, 1099. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Masui, Y.; Katsuyama, H.; Adachi, H.; Kawaguchi, A.; Hakoshima, M.; Waragai, Y.; Harigae, T.; Sako, A. An Improvement of Cardiovascular Risk Factors by Omega-3 Polyunsaturated Fatty Acids. J. Clin. Med. Res. 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartolano, F.D.C.; Dias, G.D.; Miyamoto, S.; Damasceno, N.R.T. Omega-3 Fatty Acids Improve Functionality of High-Density Lipoprotein in Individuals With High Cardiovascular Risk: A Randomized, Parallel, Controlled and Double-Blind Clinical Trial. Front. Nutr. 2022, 8, 767535. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, Y.; Koo, S.I. ATP-binding cassette transporter A1 and HDL metabolism: Effects of fatty acids. J. Nutr. Biochem. 2012, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kasbi Chadli, F.; Nazih, H.; Krempf, M.; Nguyen, P.; Ouguerram, K. Omega 3 Fatty Acids Promote Macrophage Reverse Cholesterol Transport in Hamster Fed High Fat Diet. PLoS ONE 2013, 8, e61109. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, H.J.; Yoon, J.; Hong, S.H.; Oh, C.-Y.; Lee, S.-K.; Seo, J.-M. Reversal of Intestinal Failure–Associated Liver Disease by Switching From a Combination Lipid Emulsion Containing Fish Oil to Fish Oil Monotherapy. J. Parenter. Enter. Nutr. 2016, 40, 437–440. [Google Scholar] [CrossRef]



| Variable | Control n = 10 Frequency (%) | Intervention n = 10 Frequency (%) | p Value |
|---|---|---|---|
| Sex | 0.329 | ||
| Male | 8 (80) | 6 (60) | |
| Female | 2 (20) | 4 (40) | |
| Refeeding syndrome | 0.648 | ||
| Without | 1 (10) | 2 (20) | |
| Low | 2 (20) | 3 (30) | |
| High | 7 (70) | 5 (50) | |
| Surgeries during hospital stay | 0.206 | ||
| 0 | 3 (30) | 0 (0) | |
| 1 | 4 (40) | 5 (50) | |
| 2 | 1 (10) | 4 (40) | |
| Over 3 | 2 (20) | 1 (10) | |
| Ileocecal valve | 0.136 | ||
| Presence | 10 (100) | 8 (80) | |
| Absence | 0 (0) | 2 (20) | |
| Variable | Control n = 10 | Intervention n = 10 | p Value |
| Age (years) | 45.5 ±19.6 | 52.5 ± 14.0 | 0.370 |
| Height (cm) * | 172 (163–178) | 169 (154–175) | 0.481 |
| Weight at admission (kg) | 65.5 ± 15.9 | 72.2 ± 20.2 | 0.423 |
| Usual weight (kg) | 77.1 ± 16.4 | 76.8 ± 18.4 | 0.975 |
| Ideal weight (kg) | 65.7 ± 7.6 | 63.7 ± 9.2 | 0.594 |
| Body mass index | 22.8 ± 5.95 | 26.1 ± 7.18 | 0.276 |
| Total bilirubin (mg/dL) * | 0.525 (0.365–0.710) | 0.845 (0.482–1.53) | 0.075 |
| Direct bilirubin (mg/dL) * | 0.170 (0.142–0.225) | 0.320 (0.150–0.582) | 0.089 |
| Indirect bilirubin (mg/dL) * | 0.360 (0.247–0.480) | 0.460 (0.335–0.945) | 0.190 |
| Alanine aminotransferase (U/L) * | 15.6 (13.0–19.8) | 22.8 (10.3–42.3) | 0.436 |
| Aspartate aminotransferase (U/L) * | 20.1 (14.2–27.3) | 23.5 (16.2–35.6) | 0.529 |
| Alkaline Phosphatase (U/L) | 108.5 ± 69.5 | 202 ± 109 | 0.035 |
| Total cholesterol (mg/dL) * | 77.5 (54.2–105) | 133 (81–237) | 0.114 |
| LDL cholesterol (mg/dL) * | 45.0 (20.3–67.5) | 86.5 (31–152) | 0.343 |
| HDL cholesterol (mg/dL) * | 16.5 (14.5–20.8) | 31.5 (21.5–59.5) | 0.057 |
| Triglycerides (mg/dL) * | 94.0 (89.0–196) | 131 (93.5–159) | 0.639 |
| Albumin (g/dL) * | 2.61 (2.32–3.29) | 2.78 (2.27–3.04) | 0.912 |
| Glucose (mg/dL) | 116.6 ± 35.3 | 107.8 ±16.7 | 0.486 |
| Sodium (mmol/L) | 138 ± 5.69 | 137 ± 4.87 | 0.834 |
| Potassium (mmol/L) * | 4.31 (3.77–4.41) | 4.09 (3.92–4.28) | 0.393 |
| Chloride (mmol/L) | 105 ± 6.12 | 104 ± 5.21 | 0.505 |
| Calcium (mg/dL) | 8.21 ±0.629 | 8.07 ± 0.710 | 0.658 |
| Phosphorus (mg/dL) * | 3.35 (2.51–4.17) | 3.51 (2.83–3.98) | 0.684 |
| Magnesium (mg/dL) | 2.02 ± 0.245 | 1.98 ± 0.215 | 0.711 |
| C reactive protein (mg/dL) * | 13.0 (8.66–17.1) | 7.17 (3.73–25.2) | 0.739 |
| Days from last surgery to inclusion | 29.2 ± 14.9 | 42.6 ± 26.1 | 0.248 |
| Days from admission to inclusion | 39.2 ± 18.6 | 49.7 ± 23.6 | 0.286 |
| Control n = 10 | Intervention n = 10 | p Value | |
|---|---|---|---|
| Resting energy expenditure (kcal) ^ Per body weight (kcal/kg) | 1728 ± 352 28.5 ± 6.73 | 1676 ± 416 27.0 ± 5.75 | 0.773 0.622 |
| Respiratory quotient ^ | 0.860 ±0.063 | 0.835 ± 0.065 | 0.425 |
| Oxygen volume (l) ^ | 249 ± 50.8 | 242 ± 57.9 | 0.805 |
| Carbon dioxide volume (l) ^ | 214 ±44.3 | 203 ± 58.7 | 0.671 |
| Energy from PN Total (kcal/day) Per BW (kcal/kg/day) | 1949 ± 275 32.4 ± 7.38 | 1706 ± 402 29.9 ± 8.29 | 0.131 0.479 |
| Protein from PN Total (g/day) Per BW (g/kg/day) * | 98.5 ± 15.6 1.56 (1.41–1.82) | 94.3 ± 21.4 1.44 (1.42–1.91) | 0.423 0.853 |
| Dextrose from PN Total (g/day) | 282 ± 52.7 | 243 ± 63.5 | 0.143 |
| Lipids from PN Per BW (g/kg/day) | 0.967 ± 0.207 | 0.922 ± 0.286 | 0.695 |
| n-3 PUFAs per BW (g/kg/day) | - | 0.183 ±0.078 | - |
| Carbohydrate percentage (%) * | 50.3 (49.3–52.3) | 48.5 (45.9–51.2) | 0.190 |
| Protein percentage (%) | 20.4 ± 3.33 | 21.6 ± 3.50 | 0.462 |
| Lipid percentage (%) | 30.4 ± 4.07 | 30.3 ± 3.24 | 0.986 |
| Variable | Day 0 | Day 7 | Mean Change (SD) | Difference in Adjusted Mean Change (Test—Control) | ||
|---|---|---|---|---|---|---|
| Mean (SE) | 95% CI | p Value | ||||
| MDA (nmol/mL) | ||||||
| Control (n = 10) | 2.56 ± 1.43 | 2.78 ± 1.42 | 0.219 ± 0.575 | −0.470 ± 0.291 | −1.08, 0.143 | 0.124 |
| Intervention (n = 10) | 2.04 ± 1.17 | 1.89 ± 1.15 | −0.148 ± 0.873 | |||
| GSH (nM) | ||||||
| Control (n = 10) | 1.82 ± 1.43 | 2.09 ± 1.41 | 0.266 ± 0.667 | −0.454 ± 0.219 | −0.915, 0.007 | 0.053 |
| Intervention (n = 10) | 1.86 ± 1.25 | 1.59 ± 1.01 | −0.267 ± 0.622 | |||
| GSSG (nM) | ||||||
| Control (n = 10) | 0.369 ± 0.229 | 0.371 ± 0.351 | 0.001 ± 0.230 | −0.003 ± 0.068 | −0.147, 0.142 | 0.970 |
| Intervention (n = 10) | 0.323 ± 0.265 | 0.349 ± 0.246 | 0.026 ± 0.141 | |||
| GSH/GSSG ratio | ||||||
| Control (n = 9) | 5.38 ± 7.36 | 10.9 ± 18.1 | 5.56 ± 19.0 | 0.532 ± 5.35 | −10.9, 12.0 | 0.992 |
| Intervention (n = 8) | 3.93 ± 4.00 | 5.26 ± 7.01 | 1.30 ± 4.71 | |||
| ORAC (mmoles of Trolox equivalents/mL) | ||||||
| Control (n = 10) | 2024 ± 336 | 1962 ± 263 | −62.4 ± 267 | 41.6 ± 99.9 | −169, 252 | 0.683 |
| Intervention (n = 10) | 2112 ± 562 | 2045 ± 460 | −66.9 ± 272 | |||
| Variable | Coefficient | Standard Error | p Value | 95% CI |
|---|---|---|---|---|
| Age | 0.029 | 0.012 | 0.017 | 0.005, 0.053 |
| ALT | 0.007 | 0.003 | 0.008 | 0.002, 0.012 |
| HDL-C | −0.043 | 0.018 | 0.018 | −0.078, −0.007 |
| n-3 PUFAs | −0.928 | 0.377 | 0.014 | −1.67, −0.189 |
| Variable | Day 0 | End of Follow Up | Mean Change (SD) | Difference in Adjusted Mean Change (Test—Control) | ||
|---|---|---|---|---|---|---|
| Mean (SE) | 95% CI | p Value | ||||
| Weight (kg) | ||||||
| Control (n = 10) | 62.9 ± 16.6 | 62.3 ± 15.7 | −0.630 ± 3.91 | −0.694 ± 1.59 | −2.55, 3.94 | 0.685 |
| Intervention (n = 10) | 61.4 ± 21.2 | 60.9 ± 22.0 | −0.410 ± 3.07 | |||
| BMI (kg/m2) | ||||||
| Control (n = 10) | 21.4 ± 6.34 | 21.2 ± 6.04 | −0.149 ± 1.29 | −0.208 ± 0.625 | −1.11, 1.52 | 0.625 |
| Intervention (n = 10) | 22.9 ± 8.68 | 22.8 ± 8.92 | −0.120 ± 1.52 | |||
| Muscle mass (kg) | ||||||
| Control (n = 8) | 24.7 ± 5.78 | 23.8 ±5.11 | −0.063 ± 1.30 | −1.71 ± 1.80 | −5.55, 2.13 | 0.358 |
| Intervention (n = 10) | 24.5 ± 5.37 | 22.6 ± 6.59 | −1.87 ± 4.99 | |||
| Fat mass percentage (%) | ||||||
| Control (n = 8) | 22.7 ± 18.9 | 22.9 ± 18.4 | 0.875 ± 4.64 | 0.433 ± 1.77 | −3.34, 4.21 | 0.810 |
| Intervention (n = 10) | 25.2 ± 14.7 | 26.0 ± 12.1 | 0.780 ± 5.17 | |||
| Visceral fat (cm2) | ||||||
| Control (n = 8) | 89.1 ± 88.3 | 89.2 ± 83.7 | −5.09 ± 24.1 | −1.33 ± 8.12 | −18.6, 15.9 | 0.872 |
| Intervention (n = 10) | 94.3 ± 58.5 | 89.7 ± 51.3 | −4.59 ± 24.5 | |||
| Phase angle (°) | ||||||
| Control (n = 8) | 4.53 ± 0.847 | 4.38 ± 0.888 | −0.062 ± 0.244 | −0.093 ± 0.219 | −0.560, 0.375 | 0.678 |
| Intervention (n = 10) | 4.05 ± 1.38 | 4.02 ± 1.16 | −0.030 ± 0.665 | |||
| Muscle strength | ||||||
| Control (n = 8) | 22.1 ± 12.7 | 23.6 ± 11.8 | 1.56 ± 5.72 | 0.756 ± 1.94 | −3.44, 4.95 | 0.703 |
| Intervention (n = 10) | 18.4 ± 11.8 | 17.8 ± 13.1 | 1.43 ± 2.32 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-López, A.; Guevara-Cruz, M.; Avila-Nava, A.; González-Garay, A.G.; González-Salazar, L.E.; Reyes-Ramírez, A.L.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Medina-Vera, I.; Reyes-García, J.G.; et al. n-3 Polyunsaturated Fatty Acid Supplementation Affects Oxidative Stress Marker Levels in Patients with Type II Intestinal Failure: A Randomized Double Blind Trial. Antioxidants 2023, 12, 1493. https://doi.org/10.3390/antiox12081493
Flores-López A, Guevara-Cruz M, Avila-Nava A, González-Garay AG, González-Salazar LE, Reyes-Ramírez AL, Pedraza-Chaverri J, Medina-Campos ON, Medina-Vera I, Reyes-García JG, et al. n-3 Polyunsaturated Fatty Acid Supplementation Affects Oxidative Stress Marker Levels in Patients with Type II Intestinal Failure: A Randomized Double Blind Trial. Antioxidants. 2023; 12(8):1493. https://doi.org/10.3390/antiox12081493
Chicago/Turabian StyleFlores-López, Adriana, Martha Guevara-Cruz, Azalia Avila-Nava, Alejandro G. González-Garay, Luis E. González-Salazar, Ana L. Reyes-Ramírez, José Pedraza-Chaverri, Omar N. Medina-Campos, Isabel Medina-Vera, Juan G. Reyes-García, and et al. 2023. "n-3 Polyunsaturated Fatty Acid Supplementation Affects Oxidative Stress Marker Levels in Patients with Type II Intestinal Failure: A Randomized Double Blind Trial" Antioxidants 12, no. 8: 1493. https://doi.org/10.3390/antiox12081493