Carob (Ceratonia siliqua) as Functional Feed Is Beneficial in Yellow Mealworm (Tenebrio molitor) Rearing: Evidence from Growth, Antioxidant Status and Cellular Responses
Abstract
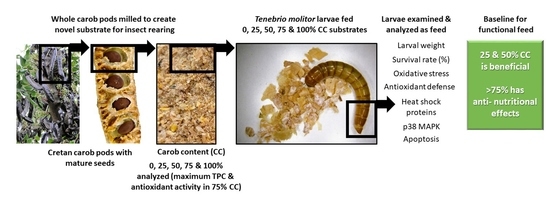
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Pilot Insect Rearing
2.3. Experimental Design
2.4. Insect Rearing for Chemical Analyses
2.5. Proximate Composition
2.6. Determination of Total Phenolic Content and Antioxidant Activity
2.6.1. Preparation of Extracts
2.6.2. Determination of Total Phenolic Content (TPC)
2.6.3. Determination of Antioxidant Activity
2.7. Assays of Antioxidant Enzymes and Lipid Peroxidation
2.8. Preparation for Immunoblotting
2.9. Statistics
3. Results
3.1. Substrates’ Proximate Composition
3.2. Growth Performance
3.2.1. Individual Larval Weight
3.2.2. Survival Rate
3.2.3. Development Time
3.3. Proximate Composition of the T. molitor Larvae
3.4. Total Phenolic Content of C. siliqua Substrates and T. molitor Larvae Extracts
3.5. Antioxidant Activity of C. siliqua Substrates and T. molitor Larvae Extracts
3.6. Lipid Peroxidation and Antioxidant Defence of T. molitor Larvae
3.7. Heat Shock Protein Response (HSP) of T. molitor Larvae
3.8. Response of T. molitor larvae p38 MAPK
3.9. Apoptosis in T. molitor Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation; Food and Agriculture Organization FAO: Rome, Italy, 2022; p. 266. [Google Scholar]
- Salter, A.M.; Lopez-Viso, C. Role of novel protein sources in sustainably meeting future global requirements. Proc. Nutr. Soc. 2021, 80, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Berggren, Å.; Jansson, A.; Low, M. Approaching ecological sustainability in the emerging insects-as-food industry. Trends Ecol. Evol. 2019, 34, 132–138. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and agriculture organization of the United Nations: Rome, Italy, 2013; p. 201. [Google Scholar]
- van Huis, A. Edible insects: Non-food and non-feed industrial applications. J. Insects Food Feed. 2022, 8, 447–450. [Google Scholar] [CrossRef]
- Finke, M.D.; Oonincx, D.G.A.B. Insects as food for insectivores. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Morales-Ramos, J.A.R., Rojas, M.G., Shapirollan, D.I., Eds.; Academic Press: London, UK, 2014; pp. 583–616. [Google Scholar]
- Bessa, L.W.; Pieterse, E.; Sigge, G.; Hoffman, L.C. Insects as human food; from farm to fork. J. Sci. Food Agric. 2020, 100, 5017–5022. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; De Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Panteli, N.; Mastoraki, M.; Rizou, E.; Stefanou, V.; Tzentilasvili, S.; Sarrou, E.; Chatzifotis, S.; Krigas, N.; Antonopoulou, E. Towards functional insect feeds: Agri-food by-products enriched with post-distillation residues of medicinal aromatic plants in Tenebrio molitor (Coleoptera: Tenebrionidae) breeding. Antioxidants 2022, 11, 68. [Google Scholar] [CrossRef]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G. A first attempt to produce proteins from insects by means of a circular economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Mastoraki, M.; Ferrándiz, P.M.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Mastoraki, M.; Katsika, L.; Enes, P.; Guerreiro, I.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. Insect meals in feeds for juvenile gilthead seabream (Sparus aurata): Effects on growth, blood chemistry, hepatic metabolic enzymes, body composition and nutrient utilization. Aquaculture 2022, 561, 738674. [Google Scholar] [CrossRef]
- Panteli, N.; Mastoraki, M.; Lazarina, M.; Chatzifotis, S.; Mente, E.; Kormas, K.A.; Antonopoulou, E. Configuration of gut microbiota structure and potential functionality in two teleosts under the influence of dietary insect meals. Microorganisms 2021, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, E.; Nikouli, E.; Piccolo, G.; Gasco, L.; Gai, F.; Chatzifotis, S.; Mente, E.; Kormas, K.A. Reshaping gut bacterial communities after dietary Tenebrio molitor larvae meal supplementation in three fish species. Aquaculture 2019, 503, 628–635. [Google Scholar] [CrossRef]
- Mente, E.; Bousdras, T.; Feidantsis, K.; Panteli, N.; Mastoraki, M.; Kormas, K.A.; Chatzifotis, S.; Piccolo, G.; Gasco, L.; Gai, F.; et al. Tenebrio molitor larvae meal inclusion affects hepatic proteome and apoptosis and/or autophagy of three farmed fish species. Scient. Rep. 2022, 7, 121. [Google Scholar] [CrossRef]
- Zohary, D. Domestication of the carob (Ceratonia siliqua L.). Isr. J. Plant Sci. 2002, 50, 141–145. [Google Scholar] [CrossRef]
- Correia, P.J.; Gama, F.; Pestana, M.; Martins-Loução, M.A. Tolerance of young (Ceratonia siliqua L.) carob rootstock to NaCl. Agric. Water Manag. 2010, 97, 910–916. [Google Scholar] [CrossRef]
- Batlle, I. Carob Tree: Ceratonia siliqua L. Promoting the Conservation and Use of Underutilized and Neglected Crops; Bioversity International: Rome, Italy, 1997; Volume 17, p. 92. [Google Scholar]
- Manso, T.; Nunes, C.; Raposo, S.; Lima-Costa, M.E. Carob pulp as raw material for production of the biocontrol agent P. agglomerans PBC-1. J. Ind. Microbiol. Biotechnol. 2010, 37, 1145–1155. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of chemical composition of carob (Ceratonia siliqua): Protein, fat, carbohydrates, and tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Grúz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.). Food Public Health 2013, 3, 304–308. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Amri, M.; Marzouki, L. Gastroprotective effect of carob (Ceratonia siliqua L.) against ethanol-induced oxidative stress in rat. BMC Complement. Med. Ther. 2015, 15, 292. [Google Scholar] [CrossRef] [PubMed]
- Abu Hafsa, S.H.; Ibrahim, S.A.; Hassan, A.A. Carob pods (Ceratonia siliqua L.) improve growth performance, antioxidant status and caecal characteristics in growing rabbits. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1307–1315. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists Inc.: Washington DC, USA, 1990. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis (apparatus, reagents, procedures, and some applications). In Agriculture Handbook United States Department of Agriculture; Agricultural Research Service USDA: Washington, DC, USA, 1970; Volume 379. [Google Scholar]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Frühbauerová, M.; Červenka, L.; Hájek, T.; Pouzar, M.; Palarčík, J. Bioaccessibility of phenolics from carob (Ceratonia siliqua L.) pod powder prepared by cryogenic and vibratory grinding. Food Chem. 2022, 377, 131968. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Emmanouilidou, M.G.; Soteriou, G.A. Asynchronous ripening behavior of cactus pear (Opuntia ficus-indica) cultivars with respect to physicochemical and physiological attributes. Food Chem. 2016, 211, 598–607. [Google Scholar] [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Salach, J.I. Preparation of monoamine oxidase from beef liver mitochondria. In Methods Enzymol.; Fleischer, S., Packer, L., Eds.; Academic Press: New York, USA, 1978; Volume 53, pp. 495–501. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods Enzymol.; Fleischer, S., Packer, L., Eds.; Academic Press: New York, NY, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione reductase. In Methods Enzymol.; Meister, A., Ed.; Academic Press: Cambridge, MA, USA, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Paoletti, F.; Mocali, A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. In Methods Enzymol.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 209–220. [Google Scholar]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.-S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Landau, S.; Or, D.; Kababya, D.; Bruckental, I.; Nitsan, Z. Analytical approach and effects of condensed tannins in carob pods (Ceratonia siliqua) on feed intake, digestive and metabolic responses of kids. Livest. Sci. 2006, 99, 29–38. [Google Scholar] [CrossRef]
- Kotrotsios, N.; Christaki, E.; Bonos, E.; Florou-Paneri, P. Dietary carob pods on growth performance and meat quality of fattening pigs. Asian-Australas. J. Anim. Sci. 2012, 25, 880–885. [Google Scholar] [CrossRef]
- Priolo, A.; Lanza, M.; Biondi, L.; Pappalardo, P.; Young, O. Effect of partially replacing dietary barley with 20% carob pulp on post-weaning growth, and carcass and meat characteristics of Comisana lambs. Meat Sci. 1998, 50, 355–363. [Google Scholar] [CrossRef]
- Sahle, M.; Coleou, J.; Haas, C. Carob pod (Ceratonia siliqua) meal in geese diets. Br. Poult. Sci. 1992, 33, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Burget, L.; Caton, S.; Bai, Y.; Spangler, L.; Gruendel, S.; Koebnick, C.; Bidlingmaier, M. Longterm effects of carob pulp preparations in insoluble fiber on metabolism, body weight and leptin levels in rats. Exp. Clin. Endocrinol. Diabetes 2007, 115, P01_124. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Baumont, R.; Noblet, J.; Renaudeau, D.; Lessire, M.; Lebas, F. Wheat Bran. Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/726 (accessed on 30 August 2022).
- Heuzé, V.; Tran, G.; Sauvant, D.; Lessire, M.; Lebas, F. Carob (Ceratonia siliqua). Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/320 (accessed on 30 August 2022).
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, N.; Benning, R. Self-selection of feeding substrates by Tenebrio molitor larvae of different ages to determine optimal macronutrient intake and the influence on larval growth and protein content. Insects 2022, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.; Gnaedinger, C.; Mélin, C. Mealworm (Tenebrio molitor). Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. Available online: https://feedipedia.org/node/16401 (accessed on 28 July 2022).
- Hichem, S.; Abdelaziz, S.; Latifa, C.; Kais, R.; Mohamed, A.; Jamel, E.-B.; Mohsen, S. In vitro and in vivo antioxidant properties of Tunisian carob (Ceratonia siliqua L.). J. Med. Plant Res. 2013, 7, 85–90. [Google Scholar] [CrossRef]
- Kogiannou, D.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, J.G.; Novotny, J.; Stejskal, V.; Laetsch, A.; Blaurock-Busch, E.; Eisenmann-Klein, M. Increased levels of transition metals in breast cancer tissue (Erratum in: Neuro Endocrinol Lett. 2007 Oct; 28(5): iii; PMID: 16804515). Neuro Endocrinol. Lett. 2006, 27, 36–39. Available online: https://pubmed.ncbi.nlm.nih.gov/16804515/ (accessed on 30 August 2022).
- Ionescu, J.G.; Merk, M.; Dowes, F. Clinical application of redox potential testing in the blood. In Proceedings of the 33rd Annual Meeting of the American Academy Environmental Medicine, Baltimore, MD, USA, 1998; pp. 503–512. [Google Scholar]
- González, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular oncology review: Ascorbic acid and cancer 25 years later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant activity and protective effect of carob honey in CCl4-induced kidney and liver injury. Arch. Med. Res. 2018, 49, 306–313. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Al-Waili, N.; El-Hilaly, J.; Al-Waili, W.; Lyoussi, B. Antioxidant, hypoglycemic, and hepatoprotective effect of aqueous and ethyl acetate extract of carob honey in streptozotocin-induced diabetic rats. Vet. World 2019, 12, 1916–1923. [Google Scholar] [CrossRef]
- Chen, J.; Xie, C.; Tian, L.; Hong, L.; Wu, X.; Han, J. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc. Nat. Acad. Sci. USA 2010, 107, 20774–20779. [Google Scholar] [CrossRef]
- Demertzioglou, M.; Antonopoulou, E.; Voutsa, D.; Kozari, A.; Moustaka-Gouni, M.; Michaloudi, E. MAPKs and HSPs’ activation of a natural Daphnia magna population in a man-perturbed lake: Implications of ecological significance. Water 2021, 13, 283. [Google Scholar] [CrossRef]
- Feidantsis, K.; Poertner, H.O.; Markou, T.; Lazou, A.; Michaelidis, B. Involvement of p38 MAPK in the induction of Hsp70 during acute thermal stress in red blood cells of the gilthead sea bream, Sparus aurata. J. Exp. Zool. A Ecol. Genet. Physiol. 2012, 317, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhan, R.; Yan, L.-C.; Gong, J.-B.; Zhao, Y.; Ma, J.; Qian, L.-J. Diet-induced elevation of circulating HSP70 may trigger cell adhesion and promote the development of atherosclerosis in rats. Cell Stress Chaperones 2016, 21, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, K.; Zhu, J.Y.; Fang, Q.; Ye, G.Y.; Wang, H.; Li, K.; Zhu, J.Y. Cloning and expression pattern of heat shock protein genes from the endoparasitoid wasp Pteromalus puparum in response to environmental stresses. Arch. Insect Biochem. Physiol. 2012, 79, 247–263. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Kentepozidou, E.; Feidantsis, K.; Roufidou, C.; Despoti, S.; Chatzifotis, S. Starvation and re-feeding affect Hsp expression, MAPK activation and antioxidant enzymes activity of European sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. A 2013, 165, 79–88. [Google Scholar] [CrossRef]
- Feidantsis, K.; Kaitetzidou, E.; Mavrogiannis, N.; Michaelidis, B.; Kotzamanis, Y.; Antonopoulou, E. Effect of taurine-enriched diets on the Hsp expression, MAPK activation and the antioxidant defence of the European sea bass (Dicentrarchus labrax). Aquacult. Nutr. 2014, 20, 431–442. [Google Scholar] [CrossRef]
- Pandey, A.; Vimal, D.; Chandra, S.; Saini, S.; Narayan, G.; Kar Chowdhuri, D. Long-term dietary exposure to low concentration of dichloroacetic acid promoted longevity and attenuated cellular and functional declines in aged Drosophila melanogaster. Age 2014, 36, 1139–1154. [Google Scholar] [CrossRef]
- Becker, J.; Craig, E.A. Heat-shock proteins as molecular chaperones. Eur. J. Biochem. 1994, 219, 11–23. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Ann. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Gobindram, M.N.N.E.; Bognanno, M.; Luciano, G.; Lanza, M.; Biondi, L. Carob pulp inclusion in lamb diets: Effect on intake, performance, feeding behaviour and blood metabolites. Anim. Prod. Sci. 2015, 56, 850–858. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Food Sci. Nutr. 2014, 3, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Bernays, E.A. Tannins: An alternative viewpoint. Entomol. Exp. Appl. 1978, 24, 244–253. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell. Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A. Anti-cancer activity and phenolic content of extracts derived from Cypriot carob (Ceratonia siliqua L.) pods using different solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef]
- Pouresmaeil, V.; Haghighi, S.; Raeisalsadati, A.S.; Neamati, A.; Homayouni-Tabrizi, M. The anti-breast cancer effects of green-synthesized zinc oxide nanoparticles using carob extracts. Anticancer Agents Med. Chem. 2021, 21, 316–326. [Google Scholar] [CrossRef] [PubMed]









| Carob Content | Dry Matter (%) | Protein (%) | Fat (%) | Ash (%) | Energy (MJ/kg) |
|---|---|---|---|---|---|
| 0% | 89.4 ± 1.5 | 16.6 ± 0.2 | 4.6 ± 0.2 | 5.1 ± 0.6 | 18.4 ± 0.1 |
| 25% | 88.6 ± 0.4 | 15.5 ± 0.5 | 4.9 ± 0.4 | 5.1 ± 0.7 | 18.4 ± 0.2 |
| 50% | 89.1 ± 0.5 | 17.2 ± 0.5 | 3.8 ± 0.2 | 6.2 ± 0.8 | 18.0 ± 0.2 |
| 75% | 89.5 ± 0.5 | 15.1 ± 0.8 | 3.2 ± 0.4 | 6.9 ± 1.2 | 17.4 ± 0.2 |
| 100% | 90.2 ± 0.3 | 14.2 ± 1.9 | 2.8 ± 0.4 | 9.0 ± 0.7 | 16.7 ± 0.2 |
| Carob Content | Dry Matter (%) | Protein Nx 6.25 (%) | Protein Nx4.76 (%) | Fat (%) | Ash (%) | Energy (MJ/kg) | Chitin (%) |
|---|---|---|---|---|---|---|---|
| 0% | 36.0 ± 1.5 | 58.9 ± 0.8 b | 44.8 ± 0.6 b | 23.8 ± 0.4 a | 5.9 ± 0.2 | 25.3 ± 0.1 a | 6.4 ± 0.0 b |
| 25% | 36.2 ± 1.2 | 61.2 ± 1.7 a,b | 46.6 ± 1.3 a,b | 23.6 ± 1.1 a,b | 4.9 ± 0.9 | 25.1 ± 0.1 a,b | 7.1 ± 0.7 b |
| 50% | 34.1 ± 1.4 | 62.1 ± 0.8 a,b | 47.3 ± 0.6 a,b | 20.7 ± 1.1 b | 4.6 ± 0.2 | 25.6 ± 0.6 a | 8.4 ± 0.2 a |
| 75% | 35.2 ± 0.2 | 64.5 ± 3.2 a | 49.1 ± 2.4 a | 23.0 ± 1.7 a,b | 4.6 ± 0.7 | 24.2 ± 0.4 b | 8.4 ± 0.4 a |
| 100% | 35.4 ± 2.0 | 64.8 ± 1.3 a | 49.4 ± 1.0 a | 21.9 ± 0.9 a,b | 4.2 ± 1.2 | 24.2 ± 0.3 b | 8.2 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonopoulou, E.; Panteli, N.; Feidantsis, K.; Mastoraki, M.; Koutsogeorgiou, E.I.; Grivaki, E.; Papagrigoriou, T.; Christias, S.P.; Chatzifotis, S.; Lazari, D.; et al. Carob (Ceratonia siliqua) as Functional Feed Is Beneficial in Yellow Mealworm (Tenebrio molitor) Rearing: Evidence from Growth, Antioxidant Status and Cellular Responses. Antioxidants 2022, 11, 1840. https://doi.org/10.3390/antiox11091840
Antonopoulou E, Panteli N, Feidantsis K, Mastoraki M, Koutsogeorgiou EI, Grivaki E, Papagrigoriou T, Christias SP, Chatzifotis S, Lazari D, et al. Carob (Ceratonia siliqua) as Functional Feed Is Beneficial in Yellow Mealworm (Tenebrio molitor) Rearing: Evidence from Growth, Antioxidant Status and Cellular Responses. Antioxidants. 2022; 11(9):1840. https://doi.org/10.3390/antiox11091840
Chicago/Turabian StyleAntonopoulou, Efthimia, Nikolas Panteli, Kostantinos Feidantsis, Maria Mastoraki, Eleni I. Koutsogeorgiou, Eirini Grivaki, Theodora Papagrigoriou, Spyros P. Christias, Stavros Chatzifotis, Diamanto Lazari, and et al. 2022. "Carob (Ceratonia siliqua) as Functional Feed Is Beneficial in Yellow Mealworm (Tenebrio molitor) Rearing: Evidence from Growth, Antioxidant Status and Cellular Responses" Antioxidants 11, no. 9: 1840. https://doi.org/10.3390/antiox11091840