Understanding the Gastrointestinal Behavior of the Coffee Pulp Phenolic Compounds under Simulated Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
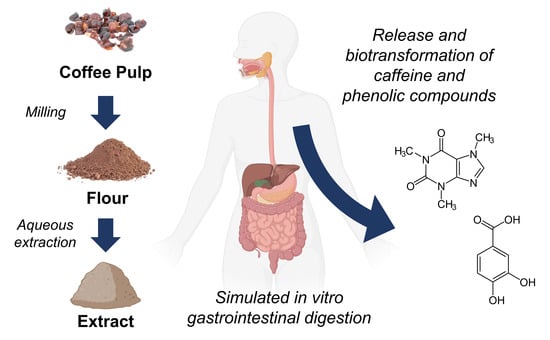
2.2. Sample Preparation
2.3. In Vitro Simulated Gastrointestinal Digestion
2.4. Extraction of Free and Bound Phenolic Compounds
2.5. Spectrophotometric Assessment of the Total Phenolic Content and Antioxidant Capacity
2.5.1. Total Phenolic Content (TPC)
2.5.2. ABTS Radical Scavenging Capacity
2.5.3. Ferric Reducing Antioxidant Power (FRAP)
2.6. HPLC-DAD-ESI/MSn Analysis of Phenolic Compounds and Methylxanthines
2.7. Retention Index and Bioaccessibility Calculation
2.8. Simulated Intestinal Absorption and Bioavailability Calculation
2.9. Simulated Colonic Gut Biotransformation
2.10. Statistical Analysis
3. Results
3.1. The Coffee Pulp Is a Source of Phenolic Compounds and Caffeine
3.2. Phenolic Compounds’ Concentration Decreased throughout the Digestion of the Coffee Pulp
3.3. Phenolic Acids and Caffeine Were Highly Bioaccessible, Whereas Flavonoids Were Degraded
3.4. Caffeine and Protocatechuic Acid Were the Main Compounds Absorbed in the Intestine after Gastrointestinal Digestion
3.5. The Matrix of the Coffee Pulp Influenced the Behavior of Phenolic Compounds during Digestion
3.6. Non-Absorbed Phenolic Compounds Might Undergo Colonic Biotransformation Yielding Small and Potentially More Adsorbable Phenolic Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- dos Santos, M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology—Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gregorio, R. Phenolic Compounds and Functional Beverages. Beverages 2021, 7, 71. [Google Scholar] [CrossRef]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Benítez, V.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Revalorization of coffee husk: Modeling and optimizing the green sustainable extraction of phenolic compounds. Foods 2021, 10, 653. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, G.E. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, G.E. Activating Effects of the Bioactive Compounds From Coffee By-Products on FGF21 Signaling Modulate Hepatic Mitochondrial Bioenergetics and Energy Metabolism in vitro. Front. Nutr. 2022, 9, 866233. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [Green Version]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz Panstw Zakl Hig. 2013, 64, 79–84. [Google Scholar] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Ben Hlel, T.; Borges, T.; Rueda, A.; Smaali, I.; Marzouki, M.N.; Seiquer, I. Polyphenols bioaccessibility and bioavailability assessment in ipecac infusion using a combined assay of simulated in vitro digestion and Caco-2 cell model. Int. J. Food Sci. Technol. 2019, 54, 1566–1575. [Google Scholar] [CrossRef]
- Gonçalves, J.; Ramos, R.; Luís, Â.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Wang, N.N.; Dong, J.; Deng, Y.H.; Zhu, M.F.; Wen, M.; Yao, Z.J.; Lu, A.P.; Wang, J.B.; Cao, D.S. ADME Properties Evaluation in Drug Discovery: Prediction of Caco-2 Cell Permeability Using a Combination of NSGA-II and Boosting. J. Chem. Inf. Model. 2016, 56, 763–773. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-De-La-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.; Fanti, F.; Palmieri, S.; Viteritti, E.; Eugelio, F.; Pepe, A.; Compagnone, D.; Sergi, M. Predictive Multi Experiment Approach for the Determination of Conjugated Phenolic Compounds in Vegetal Matrices by Means of LC-MS/MS. Molecules 2022, 27, 3089. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 461. [Google Scholar] [CrossRef]
- Cañas, S.; Rebollo-Hernanz, M.; Cano-Muñoz, P.; Aguilera, Y.; Bení Tez, V.; Braojos, C.; Gila-Dí Az, A.; Rodríguez-Rodríguez, P.; Cobeta, I.M.; López De Pablo, Á.L.; et al. Critical Evaluation of Coffee Pulp as an Innovative Antioxidant Dietary Fiber Ingredient: Nutritional Value, Functional Properties and Acute and Sub-Chronic Toxicity. Proceedings 2021, 70, 65. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Fernández-Gómez, B.; Herrero, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Uribarri, J.; Del Castillo, M.D. Inhibition of the Maillard reaction by phytochemicals composing an aqueous coffee silverskin extract via a mixed mechanism of action. Foods 2019, 8, 438. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Arch. Latinoam. Nutr. 2016, 66, 87–100. [Google Scholar]
- Furia, E.; Beneduci, A.; Malacaria, L.; Fazio, A.; La Torre, C.; Plastina, P. Modeling the solubility of phenolic acids in aqueous media at 37 °C. Molecules 2021, 26, 6500. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Mosele, J.I.; Motilva, M.J.; Ludwig, I.A. Beta-Glucan and Phenolic Compounds: Their Concentration and Behavior during in Vitro Gastrointestinal Digestion and Colonic Fermentation of Different Barley-Based Food Products. J. Agric. Food Chem. 2018, 66, 8966–8975. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Corona-Leo, L.S.; Meza-Márquez, O.G.; Hernández-Martínez, D.M. Effect of in vitro digestion on phenolic compounds and antioxidant capacity of different apple (Malus domestica) varieties harvested in Mexico. Food Biosci. 2021, 43, 101311. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Qin, W.; Ketnawa, S.; Ogawa, Y. Effect of digestive enzymes and pH on variation of bioavailability of green tea during simulated in vitro gastrointestinal digestion. Food Sci. Hum. Wellness 2022, 11, 669–675. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kanellos, P.T.; Kalogeropoulos, N. Gallic acid bioavailability in humans. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Nova Science Publisher: Hauppauge, NY, USA, 2013; pp. 301–312. ISBN 9781626189218. [Google Scholar]
- Zheng, J.; Xiong, H.; Li, Q.; He, L.; Weng, H.; Ling, W.; Wang, D. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Sci. Nutr. 2019, 7, 3071–3080. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 710–737. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Kelly, S.M.; O’Callaghan, J.; Kinsella, M.; Van Sinderen, D. Characterisation of a hydroxycinnamic acid esterase from the bifidobacterium longumsubsp. Longumtaxon. Front. Microbiol. 2018, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Grzelczyk, J.; Szwajgier, D.; Baranowska-Wójcik, E.; Budryn, G.; Zakłos-Szyda, M.; Sosnowska, B. Bioaccessibility of coffee bean hydroxycinnamic acids during in vitro digestion influenced by the degree of roasting and activity of intestinal probiotic bacteria, and their activity in Caco-2 and HT29 cells. Food Chem. 2022, 392, 133328. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Zapata, F.J.; Rebollo-Hernanz, M.; Novakofski, J.E.; Nakamura, M.T.; de Mejia, E.G. Caffeine, but not other phytochemicals, in mate tea (Ilex paraguariensis St. Hilaire) attenuates high-fat-high-sucrose-diet-driven lipogenesis and body fat accumulation. J. Funct. Foods 2020, 64, 103646. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Olmo-García, L.; Figueiredo-González, M.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B. Application of the INFOGEST Standardized Method to Assess the Digestive Stability and Bioaccessibility of Phenolic Compounds from Galician Extra-Virgin Olive Oil. J. Agric. Food Chem. 2021, 69, 11592–11605. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic metabolism of flavonoids by gut microbiota and its impact on gastrointestinal cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]

 ) to the highest (
) to the highest ( ) value for each parameter) (B) illustrating the behavior of phenolic compounds and caffeine from the coffee pulp during simulated gastrointestinal digestion.
) value for each parameter) (B) illustrating the behavior of phenolic compounds and caffeine from the coffee pulp during simulated gastrointestinal digestion.
 ) to the highest (
) to the highest ( ) value for each parameter) (B) illustrating the behavior of phenolic compounds and caffeine from the coffee pulp during simulated gastrointestinal digestion.
) value for each parameter) (B) illustrating the behavior of phenolic compounds and caffeine from the coffee pulp during simulated gastrointestinal digestion.

| Comp. | Rt (min) | λmax (nm) | Molecular Ion [M-H]− (m/z) | Fragments MS2 | Tentative Identification | Common Name |
|---|---|---|---|---|---|---|
| 1 | 4.38 | 270 | 169 | – | 3,4,5-trihydroxybenzoic acid | Gallic acid |
| 2 | 5.17 | 326 | 353 | 191(100), 135(83), 179(54), 173(5), 161(7) | 3-O-caffeoyl quinic acid | Chlorogenic acid |
| 3 | 6.08 | 260, 294 | 153 | 109(70) | 3,4-dihydroxybenzoic acid | Protocatechuic acid |
| 4 | 7.28 | 326 | 353 | 191(8), 179(4), 173(3), 161(12), 135(94) | 4-O-caffeoyl quinic acid cis isomer | Cryptochlorogenic acid (cis) |
| 5 | 8.12 | 326 | 353 | 191(100), 179(18), 173(6), 161(21), 135(12) | 4-O-caffeoyl quinic acid trans isomer | Cryptochlorogenic acid (trans) |
| 6 | 10.05 | 328 | 353 | 191(39), 179(9), 173(49), 155(33), 135(38) | 5-O-caffeoyl quinic acid | Neochlorogenic acid |
| 7 | 11.35 | 252, 290 | 167 | – | 4-hydroxy-3-methoxybenzoic acid | Vanillic acid |
| 8 | 11.70 | 323 | 179 | – | 3,4-dihydroxycinnamic acid | Caffeic acid |
| 9 | 12.99 | 275 | – | – | 1,3,7-trimethylxanthine | Caffeine |
| 10 | 12.30 | 340 | 593 | 353(100), 383(70), 297(55), 473(50), 503(15) | Apigenin 6,8-di-C-glucoside | Vicenin-2 |
| 11 | 16.36 | 355 | 625 | 301(100) | Quercetin 3,7-dihexoside | – |
| 12 | 16.60 | 328 | 367 | 193(6), 191(100), 173(4), 134(9) | 5-feruloylquinic acid | – |
| 13 | 17.40 | 233, 314 | 163 | 119(70) | 4-hydroxycinnamic acid | p-coumaric acid |
| 14 | 19.80 | 356 | 609 | 301(100) | Quercetin-3-O-rutinoside | Rutin |
| 15 | 21.77 | 328 | 515 | 353(100), 335(5), 191(97), 179(79), 173(9), 161(5), 135(37) | 3,5-dicaffeoylquinic acid | Isochlorogenic acid A |
| 16 | 21.81 | 356 | 463 | 301(100) | Quercetin-3-O-glucoside | Isoquercetin |
| 17 | 23.49 | 314 | 337 | 191(100), 173(10), 163(69) | 5-p-coumaroylquinic acid | – |
| Compounds | ND | OP | GP | IP | CP | C2A | HIA |
|---|---|---|---|---|---|---|---|
| Coffee pulp flour | |||||||
| Hydroxybenzoic acid derivatives | |||||||
| Gallic acid | 469.4 ± 20.2 a | 281.9 ± 24.0 b | 249.7 ± 11.7 b | 143.8 ± 20.1 c | 103.9 ± 13.0 d | 77.5 ± 10.8 de | 62.2 ± 8.7 e |
| Protocatechuic acid | 1757.5 ± 7.3 a | 1079.2 ± 19.6 d | 1315.6 ± 81.9 bcd | 1615.8 ± 239.5 ab | 1089.2 ± 117.1 cd | 1411.4 ± 209.3 abc | 1020.1 ± 151.2 d |
| Vanillic acid | nd | nd | nd | nd | 7.6 ± 0.3 a | – | – |
| Hydroxycinnamic acid derivatives | |||||||
| Chlorogenic acid | 52.8 ± 6.3 b | 29.8 ± 1.9 d | 40.2 ± 5.1 c | 65.0 ± 3.9 a | 57.5 ± 5.1 ab | 9.9 ± 0.6 e | 22.4 ± 1.4 d |
| Cryptochlorogenic acid (cis) | 66.2 ± 5.0 bc | 41.4 ± 2.0 d | 58.5 ± 5.1 c | 81.2 ± 12.4 ab | 94.0 ± 12.7 a | 12.3 ± 1.9 e | 21.3 ± 3.3 e |
| Cryptochlorogenic acid (trans) | 853.2 ± 14.3 a | 459.1 ± 5.9 c | 635.1 ± 42.4 b | 676.7 ± 73.4 b | 180.3 ± 20.9 d | 69.7 ± 7.6 d | 177.7 ± 19.3 d |
| Neochlorogenic acid | 38.9 ± 2.7 a | 15.4 ± 1.0 c | 12.8 ± 1.7 cd | 27.7 ± 3.6 b | nd | 4.2 ± 0.5 e | 9.5 ± 1.2 d |
| Caffeic acid | nd | nd | nd | nd | 110.0 ± 15.3 a | – | – |
| 5-feruloylquinic acid | 20.1 ± 1.3 a | nd | 13.8 ± 0.5 b | nd | nd | – | – |
| p-coumaric acid | nd | nd | nd | nd | 50.6 ± 7.8 a | – | – |
| Isochlorogenic acid A | 49.0 ± 1.1 a | 14.2 ± 1.5 d | 22.1 ± 2.0 c | 38.6 ± 4.2 b | 20.2 ± 2.6 c | 3.9 ± 0.4 e | 13.2 ± 1.4 d |
| 5-p-coumaroylquinic acid | 28.5 ± 0.0 a | 1.9 ± 0.7 e | 5.6 ± 0.7 cd | 23.3 ± 3.4 b | 4.2 ± 0.4 de | 5.7 ± 0.8 cd | 8.9 ± 1.3 c |
| Flavones | |||||||
| Vicenin-2 | 64.3 ± 2.0 a | 17.9 ± 0.4 d | 34.3 ± 3.5 b | 26.9 ± 3.5 c | 20.1 ± 1.6 d | 3.2 ± 0.4 e | 5.0 ± 0.7 e |
| Flavonols | |||||||
| Quercetin 3,7-dihexoside | 25.9 ± 1.1 a | 11.8 ± 0.8 c | 17.1 ± 0.1 b | 16.3 ± 1.3 b | nd | 1.8 ± 0.1 d | 1.2 ± 0.1 d |
| Rutin | 79.3 ± 2.1 a | 34.1 ± 1.1 d | 54.8 ± 6.0 b | 46.3 ± 4.8 c | nd | 6.3 ± 0.7 e | 10.3 ± 1.1 e |
| Isoquercetin | 60.9 ± 2.6 a | 18.7 ± 1.4 c | 30.8 ± 4.0 b | 34.9 ± 5.3 b | 20.7 ± 2.4 c | 15.4 ± 2.3 cd | 11.1 ± 1.7 d |
| Methylxanthines | |||||||
| Caffeine | 4730.6 ± 60.9 a | 2013.2 ± 0.1 e | 3009.3 ± 314.8 d | 3887.1 ± 220.6 b | 3618.9 ± 29.8 bc | 3805.5 ± 215.9 bc | 3462.8 ± 196.5 c |
| Coffee pulp extract | |||||||
| Hydroxybenzoic acid derivatives | |||||||
| Gallic acid | 684.5 ± 40.4 a | 759.5 ± 78.1 a | 466.0 ± 28.5 b | 222.9 ± 23.3 c | 666.4 ± 74.0 a | 120.1 ± 12.5 cd | 96.4 ± 10.1 d |
| Protocatechuic acid | 3132.1 ± 75.2 a | 3266.6 ± 111.8 a | 3037.3 ± 281.7 a | 2212.7 ± 258.2 b | 2338.4 ± 294.1 b | 1932.9 ± 225.5 b | 1397.0 ± 163.0 c |
| Hydroxycinnamic acid derivatives | |||||||
| Chlorogenic acid | 120.9 ± 9.9 a | 121.6 ± 23.0 a | 124.2 ± 14.1 a | 104.5 ± 14.4 a | nd | 15.9 ± 2.2 b | 36.0 ± 5.0 b |
| Cryptochlorogenic acid (cis) | 127.4 ± 5.7 b | 155.1 ± 19.2 a | 137.4 ± 9.5 ab | 145.0 ± 4.3 ab | nd | 22.0 ± 0.7 c | 38.1 ± 1.1 c |
| Cryptochlorogenic acid (trans) | 1450.0 ± 3.7 a | 1508.2 ± 21.0 a | 1137.9 ± 97.0 b | 651.6 ± 7.6 c | 231.1 ± 8.3 de | 67.2 ± 0.8 f | 171.2 ± 2.0 ef |
| Caffeic acid | nd | nd | nd | nd | 1684.2 ± 201.7 a | – | – |
| 5-feruloylquinic acid | 86.7 ± 1.4 ab | 81.1 ± 4.0 b | 96.8 ± 7.8 a | nd | nd | – | – |
| p-coumaric acid | nd | nd | nd | nd | 22.6 ± 2.1 a | – | – |
| 3,5-dicaffeoylquinic acid | 70.3 ± 1.3 a | 73.5 ± 8.1 a | 67.4 ± 4.7 a | 68.7 ± 7.9 a | t | 7.0 ± 0.8 c | 23.5 ± 2.7 b |
| 5-p-coumaroylquinic acid | t | t | t | t | t | – | – |
| Flavones | |||||||
| Vicenin-2 | 80.3 ± 11.3 a | 70.3 ± 10.9 a | 69.5 ± 8.3 a | nd | nd | – | – |
| Flavonols | |||||||
| Rutin | 145.4 ± 2.0 a | 148.7 ± 8.7 a | 138.2 ± 12.8 a | nd | t | – | – |
| Isoquercetin | 89.4 ± 2.0 ab | 98.8 ± 6.5 a | 85.4 ± 8.3 b | t | t | – | – |
| Methylxanthines | |||||||
| Caffeine | 7879.5 ± 343.4 abc | 8339.0 ± 298.3 a | 8137.1 ± 852.2 ab | 6689.4 ± 700.9 bcd | 6539.4 ± 932.8 cd | 6549.0 ± 686.1 cd | 5959.3 ± 624.4 d |
| Compounds | Retention Index | Bioaccessibility | Bioavailability | |||
|---|---|---|---|---|---|---|
| OP | GP | IP | CP | C2A | HIA | |
| Coffee pulp flour | ||||||
| Hydroxybenzoic acid derivatives | ||||||
| Gallic acid | 60.1 ± 7.7 a** | 53.2 ± 4.8 a* | 30.6 ± 5.6 b | 22.1 ± 5.6 bc*** | 16.5 ± 3.0 c | 13.2 ± 2.4 c |
| Protocatechuic acid | 61.4 ± 1.4 bc*** | 74.9 ± 5.0 abc* | 91.9 ± 14.0 a | 62.0 ± 6.9 bc | 80.3 ± 12.2 ab* | 58.0 ± 8.8 c* |
| Hydroxycinnamic acid derivatives | ||||||
| Chlorogenic acid | 56.6 ± 10.3 c* | 76.2 ± 18.9 bc | 123.2 ± 22.2 a* | 109.0 ± 22.8 ab | 18.8 ± 3.4 d* | 42.4 ± 7.6 cd* |
| Cryptochlorogenic acid (cis) | 62.6 ± 7.7 cd** | 88.4 ± 14.4 bc | 122.7 ± 28.1 ab | 142.1 ± 29.9 a | 18.6 ± 4.2 e | 32.2 ± 7.4 de |
| Cryptochlorogenic acid (trans) | 53.8 ± 1.6 b*** | 74.4 ± 6.2 a | 79.3 ± 9.9 a*** | 21.1 ± 2.8 c* | 8.2 ± 1.0 c*** | 20.8 ± 2.6 c*** |
| Neochlorogenic acid | 39.7 ± 5.3 b | 33.0 ± 6.6 b | 71.2 ± 14.2 a | – | 10.8 ± 2.2 c | 24.5 ± 4.9 bc |
| 5-feruloylquinic acid | – | 68.6 ± 6.8 a*** | – | – | – | – |
| 3,5-dicaffeoylquinic acid | 29.1 ± 3.6 cd*** | 45.0 ± 5.1 b*** | 78.9 ± 10.3 a | 41.2 ± 6.3 bc | 8.0 ± 1.1 e | 27.0 ± 3.5 d |
| 5-p-coumaroylquinic acid | 6.7 ± 1.0 d | 19.7 ± 2.5 bc | 81.8 ± 12.0 a | 14.7 ± 1.3 cd | 20.2 ± 3.0 bc | 31.1 ± 4.6 b |
| Flavones | ||||||
| Vicenin-2 | 27.8 ± 1.5 c** | 53.3 ± 7.1 a* | 41.9 ± 6.8 b | 31.3 ± 3.5 c | 5.0 ± 0.8 d | 7.8 ± 3.1 d |
| Flavonols | ||||||
| Quercetin 3,7-dihexoside | 45.4 ± 5.0 b | 66.1 ± 3.3 a | 62.8 ± 7.7 a | – | 6.9 ± 0.8 c | 4.7 ± 0.6 c |
| Rutin | 43.0 ± 2.5 b*** | 69.0 ± 9.4 a** | 58.4 ± 7.6 a | – | 8.0 ± 1.0 c | 13.0 ± 1.7 c |
| Isoquercetin | 30.7 ± 3.5 bc*** | 50.6 ± 8.8 a*** | 57.3 ± 11.1 a | 34.0 ± 5.4 b | 25.3 ± 4.9 bc | 18.2 ± 3.5 bc |
| Methylxanthines | ||||||
| Caffeine | 42.6 ± 0.5 c*** | 63.6 ± 7.5 b*** | 82.2 ± 5.7 a | 76.5 ± 1.6 a | 80.4 ± 5.6 a | 72.2 ± 5.1 ab |
| Coffee pulp extract | ||||||
| Hydroxybenzoic acid derivatives | ||||||
| Gallic acid | 111.0 ± 18.0 a | 68.1 ± 8.2 b | 32.6 ± 5.3 c | 97.4 ± 16.6 a | 17.5 ± 2.9 c | 14.1 ± 2.3 c |
| Protocatechuic acid | 104.3 ± 6.1 a | 97.0 ± 11.3 a | 70.6 ± 9.9 b | 74.7 ± 11.2 b | 61.7 ± 8.7 b | 44.6 ± 6.3 c |
| Hydroxycinnamic acid derivatives | ||||||
| Chlorogenic acid | 100.6 ± 27.2 a | 102.8 ± 20.1 a | 86.5 ± 18.9 a | – | 13.2 ± 2.9 b | 29.8 ± 6.5 b |
| Cryptochlorogenic acid (cis) | 121.7 ± 20.5 a | 107.9 ± 12.3 a | 113.8 ± 8.5 a | – | 17.2 ± 1.3 b | 29.9 ± 2.2 b |
| Cryptochlorogenic acid (trans) | 104.0 ± 1.7 a | 78.5 ± 6.9 a | 44.9 ± 0.6 c | 15.9 ± 0.6 c | 4.6 ± 0.1 d | 11.8 ± 0.2 cd |
| 5-feruloylquinic acid | 93.5 ± 6.1 a | 111.6 ± 10.7 a | – | – | – | – |
| 3,5-dicaffeoylquinic acid | 104.6 ± 13.4 a | 95.8 ± 8.4 a | 97.7 ± 13.1 a | – | 10.0 ± 1.3 c | 33.4 ± 4.5 b |
| Flavones | ||||||
| Vicenin-2 | 87.5 ± 25.8 a | 86.5 ± 22.5 a | – | – | – | – |
| Flavonols | ||||||
| Rutin | 102.3 ± 7.4 a | 95.1 ± 10.1 a | – | – | – | – |
| Isoquercetin | 110.6 ± 9.7 a | 95.6 ± 11.4 a | – | – | – | – |
| Methylxanthines | ||||||
| Caffeine | 105.8 ± 8.4 a | 103.3 ± 15.3 ab | 84.9 ± 12.6 ab | 83.0 ± 15.5 ab | 83.1 ± 12.3 ab | 75.6 ± 11.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañas, S.; Rebollo-Hernanz, M.; Braojos, C.; Benítez, V.; Ferreras-Charro, R.; Dueñas, M.; Aguilera, Y.; Martín-Cabrejas, M.A. Understanding the Gastrointestinal Behavior of the Coffee Pulp Phenolic Compounds under Simulated Conditions. Antioxidants 2022, 11, 1818. https://doi.org/10.3390/antiox11091818
Cañas S, Rebollo-Hernanz M, Braojos C, Benítez V, Ferreras-Charro R, Dueñas M, Aguilera Y, Martín-Cabrejas MA. Understanding the Gastrointestinal Behavior of the Coffee Pulp Phenolic Compounds under Simulated Conditions. Antioxidants. 2022; 11(9):1818. https://doi.org/10.3390/antiox11091818
Chicago/Turabian StyleCañas, Silvia, Miguel Rebollo-Hernanz, Cheyenne Braojos, Vanesa Benítez, Rebeca Ferreras-Charro, Montserrat Dueñas, Yolanda Aguilera, and María A. Martín-Cabrejas. 2022. "Understanding the Gastrointestinal Behavior of the Coffee Pulp Phenolic Compounds under Simulated Conditions" Antioxidants 11, no. 9: 1818. https://doi.org/10.3390/antiox11091818