Applications of Antioxidants in Dental Procedures
Abstract
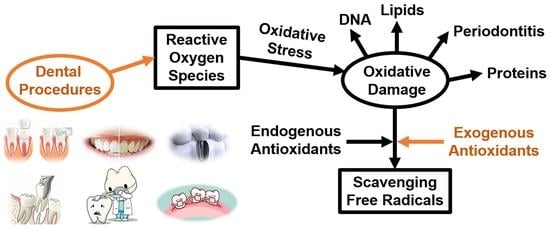
:1. Introduction
2. Reactive Oxygen Species (ROS) and Oxidative Damage
2.1. ROS in Oral Environment
2.2. Oxidative Stress and Oxidative Damage
3. Antioxidants Used in Dental Procedures
3.1. Tooth Bleaching
3.2. Dental Implants
3.3. Dental Restorations
3.3.1. Dental Amalgam
3.3.2. Dental Resin Composites
3.3.3. Glass-Ionomer Cement (GIC)
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rozier, R.G.; Pahel, B.T. Patient-and population-reported outcomes in public health dentistry: Oral health-related quality of life. Dent. Clin. N. Am. 2008, 52, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Carnelio, S.; Khan, S.A.; Rodrigues, G. Definite, probable or dubious: Antioxidants trilogy in clinical dentistry. Br. Dent. J. 2008, 204, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Hendi, S.S.; Kasraei, S.; Moghimbeigi, A. Total antioxidant capacity of saliva and dental caries. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e553. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksakalli, S. Antioxidants in dentistry: Review of literature. Dentistry 2013, 4, 1000181. [Google Scholar] [CrossRef] [Green Version]
- King, M.; Chatelain, K.; Farris, D.; Jensen, D.; Pickup, J.; Swapp, A.; O’Malley, S.; Kingsley, K. Oral squamous cell carcinoma proliferative phenotype is modulated by proanthocyanidins: A potential prevention and treatment alternative for oral cancer. BMC Complement Altern. Med. 2007, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Shirataki, Y.; Kawase, M.; Saito, S.; Kurihara, T.; Tanaka, W.; Satoh, K.; Sakagami, H.; Motohashi, N. Selective cytotoxic activity of grape peel and seed extracts against oral tumor cell lines. Anticancer Res. 2000, 20, 423–426. [Google Scholar]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun., W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol-gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Lee, D.W.; Jung, E.J.; Bae, J.T.; Lee, S.G.; Pyo, H.B.; Kang, K.H.; Lee, D.K. Preparation and characterization of quercetin-loaded silica microspheres stabilized by combined multiple emulsion and sol-gel processes. Chem. Ind. Chem. Eng. Q. 2015, 21, 85–94. [Google Scholar] [CrossRef]
- Skibsted, L.H.; Dragsted, L.O.; Dyerberg, J.; Hansen, H.S.; Tjnneland, A.M. Antioxidants and health. Ugeskr. Laeg. 2006, 168, 2787–2789. [Google Scholar] [PubMed]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef] [PubMed]
- Pua, M.L.; Yoshitomi, T.; Chonpathompikunlert, P.; Hirayama, A.; Nagasaki, Y. Redox-active injectable gel using thermo-responsive nanoscale polyion complex flower micelle for noninvasive treatment of local inflammation. J. Control Release 2013, 172, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation of a starch-based carrier for oral delivery of Vitamin E to the small intestine. Food Hydrocoll. 2019, 91, 26–33. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Deng, M.; Bai, Y.; Chen, X.; Jing, X. Biodegradable thermo-and pH-responsive hydrogels for oral drug delivery. J. Polym. Sci. A Polym. Chem. 2011, 49, 2941–2951. [Google Scholar] [CrossRef]
- Das, S.S.; Sarkar, A.; Chabattula, S.C.; Verma, P.R.P.; Nazir, A.; Gupta, P.K.; Ruokolainen, J.; Kesari, K.K.; Singh, S.K. Food-grade quercetin-loaded nanoemulsion ameliorates effects associated with parkinson’s disease and cancer: Studies employing a transgenic c. elegans model and human cancer cell lines. Antioxidants 2022, 11, 1378. [Google Scholar] [CrossRef]
- Das, S.S.; Verma, P.R.P.; Singh, S.K. Screening and preparation of quercetin doped nanoemulsion: Characterizations, antioxidant and anti-bacterial activities. LWT-Food Sci. Technol. 2020, 124, 109141. [Google Scholar] [CrossRef]
- Vidhya, S.; Srinivasulu, S.; Sujatha, M.; Mahalaxmi, S. Effect of grape seed extract on the bond strength of bleached enamel. Oper. Dent. 2011, 36, 433–438. [Google Scholar] [CrossRef]
- Tian, Y.; Mao, X.; Sun, R.; Zhang, M.; Xia, Q. Enhanced oral bioavailability of oligomeric proanthocyanidins by a self-double-emulsifying drug delivery system. Food Sci. Nutr. 2020, 8, 3814–3825. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Hong, T.; Xie, J.; Zhan, X.; Wang, Y. Application of reactive oxygen species-based nanomaterials in dentistry: A review. Crystals 2021, 11, 266. [Google Scholar] [CrossRef]
- Tothova, L.U.; Celec, P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- D’aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246. [Google Scholar] [CrossRef] [Green Version]
- Waszkiewicz, N.; Chojnowska, S.; Zalewska, A.; Zwierz, K.; Szulc, A.; Szajda, S.D. Salivary hexosaminidase in smoking alcoholics with bad periodontal and dental states. Drug Alcohol Depend. 2013, 129, 33–40. [Google Scholar] [CrossRef]
- Kimura, M.; Miyakawa, T.; Matsushita, S.; So, M.; Higuchi, S. Gender differences in the effects of ADH1B and ALDH2 polymorphisms on alcoholism. Alcohol. Clin. Exp. Res. 2011, 35, 1923–1927. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Klein, I.; Eiserich, J.P.; Cross, C.E.; Nagler, R.M. Inhibition of oral peroxidase activity by cigarette smoke: In vivo and in vitro studies. Free Radic. Biol. Med. 2003, 34, 377–384. [Google Scholar] [CrossRef]
- Sim, S.S.; Choi, J.C.; Rhie, D.J.; Yoon, S.H.; Hahn, S.J.; Kim, C.J.; Kim, M.S.; Jo, Y.H. The involvement of phospholipase A2 in ethanol-induced gastric muscle contraction. Eur. J. Pharmacol. 2001, 413, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J. Effects of cigarette smoke on the lung and systemic immunity. J. Physiol. Pharmacol. 2008, 59, 19–34. [Google Scholar] [PubMed]
- Swan, G.E.; Lessov-Schlaggar, C.N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007, 17, 259–273. [Google Scholar] [CrossRef]
- Kanehira, T.; Shibata, K.; Kashiwazaki, H.; Inoue, N.; Morita, M. Comparison of antioxidant enzymes in saliva of elderly smokers and non-smokers. Gerodontology 2006, 23, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, U.; Maciejczyk, M.; Miąsko, A.; Matczuk, J.; Knaś, M.; Żukowski, P.; Żendzian-Piotrowska, M.; Borys, J.; Zalewska, A. Oxidative modification in the salivary glands of high fat-diet induced insulin resistant rats. Front. Physiol. 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopáni, M.; Celec, P.; Danišovič, L.; Michalka, P.; Biró, C. Oxidative stress and electron spin resonance. Clin. Chim. Acta 2006, 364, 61–66. [Google Scholar] [CrossRef]
- Kna, M.; Maciejczyk, M.; Waszkiel, D.; Zalewska, A. Oxidative stress and salivary antioxidants. Dent. Med. Probl. 2013, 50, 461–466. [Google Scholar]
- Esterbauer, H.; Puhl, H.; Dieber-rotheneder, M.; Waeg, G.; Rabl, H. Effect of antioxidants on oxidative modification of LDL. Ann. Med. 1991, 23, 573–581. [Google Scholar] [CrossRef]
- TAAo, P. Parameter on chronic periodontitis with slight to moderate loss of periodontal support. J. Periodontol 2000, 71, 853–855. [Google Scholar]
- Kornman, K.S. Mapping the pathogenesis of periodontitis: A new look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef]
- Muniz, F.W.M.G.; Nogueira, S.B.; Mendes, F.L.V.; Rösing, C.K.; Moreira, M.M.S.M.; de Andrade, G.M.; de Sousa Carvalho, R. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch. Oral Biol. 2015, 60, 1203–1214. [Google Scholar] [CrossRef]
- Junior, M.T.; Rodrigues, C.A.; Bernardes, V.L.; de Araujo, T.S.B.; Nicoli, G.A.; dos Reis Derceli, J. Dental bleaching and new possibilities: Literature review. Health Sci. J. 2018, 12, 600. [Google Scholar] [CrossRef]
- Lertsukprasert, N.; Locharoenrat, K. Efficiency of tooth bleaching agent on staining and discoloration characteristics of nicotine stained dental enamel model. BMC Oral Health 2020, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, S.W.; Heithersay, G.S.; Bridges, T.E. Hydroxyl radical activity in thermo-catalytically bleached root-filled teeth. Dent. Traumatol. 1997, 13, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Tsujimoto, Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J. Endod. 2004, 30, 45–50. [Google Scholar] [CrossRef] [PubMed]
- De Moor, R.J.G.; Diachuk, A.; Verheyen, P.; Meire, M.A.; De Coster, P.J.; Keulemans, F.; De Bruyne, M.; Walsh, L.J. Insight in the chemistry of laser-activated dental bleaching. Sci. World J. 2015, 2015, 650492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Concentrations of and application protocols for hydrogen peroxide bleaching gels: Effects on pulp cell viability and whitening efficacy. J. Dent. 2014, 42, 185–198. [Google Scholar] [CrossRef]
- Nathanson, D. Vital tooth bleaching: Sensitivity and pulpal considerations. J. Am. Dent. Assoc. 1997, 128, 41S–44S. [Google Scholar] [CrossRef]
- Rotstein, I.; Torek, Y.; Misgav, R. Effect of cementum defects on radicular penetration of 30% H2O2 during intracoronal bleaching. J. Endod. 1991, 17, 230–233. [Google Scholar] [CrossRef]
- Anugrahati, D.F.; Ernawati, R.; Irsya, W.; Sumur, Y.K.; Sukaton; Ismiyati, K. Management of non-vital teeth discoloration with the internal bleaching: A case report. Indian J. Forensic Med. Toxicol. 2021, 15, 1072–1076. [Google Scholar]
- Curran, S.F.; Amoruso, M.A.; Goldstein, B.D.; Berg, R.A. Degradation of soluble collagen by ozone or hydroxyl radicals. FEBS Lett. 1984, 176, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, D.; Halliwell, B. Formation of hydroxyl radicals from hydrogen peroxide and iron salts by superoxide-and ascorbate-dependent mechanisms: Relevance to the pathology of rheumatoid disease. Clin. Sci. 1983, 64, 649–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutteridge, J.M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS let. 1986, 201, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Free radicals, reactive oxygen species and human disease: A critical evaluation with special reference to atherosclerosis. Br. J. Exp. Pathol. 1989, 70, 737. [Google Scholar] [PubMed]
- Titley, K.; Torneck, C.D.; Ruse, N.D. The effect of carbamide-peroxide gel on the shear bond strength of a microfil resin to bovine enamel. J. Dent. Res. 1992, 71, 20–24. [Google Scholar] [CrossRef]
- Dishman, M.V.; Covey, D.A.; Baughan, L.W. The effects of peroxide bleaching on composite to enamel bond strength. Dent. Mater. J. 1994, 10, 33–36. [Google Scholar] [CrossRef]
- Titley, K.C.; Torneck, C.D.; Ruse, N.D.; Krmec, D. Adhesion of a resin composite to bleached and unbleached human enamel. J. Endod. 1993, 19, 112–115. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Koga, A.F.; Borges, A.B. The effects of anti-oxidant agents as neutralizers of bleaching agents on enamel bond strength. Braz. J. Oral Sci. 2006, 5, 971–976. [Google Scholar]
- Oskoee, P.A.; Navimipour, E.J.; Oskoee, S.S.; Moosavi, N. Effect of 10% sodium ascorbate on bleached bovine enamel surface morphology and microhardness. Open Dent. J. 2010, 4, 207. [Google Scholar] [CrossRef] [Green Version]
- Bulut, H.; Turkun, M.; Kaya, A.D. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am. J. Orthod. 2006, 129, 266–272. [Google Scholar] [CrossRef]
- Çakir, E.G.; Özcan, S.; Tulunoglu, I.; Üçtaşli, M.B.; Tulunoglu, O. Efficacy of In-office Bleaching on Microhardness of Permanent Teeth with Antioxidant Re-hardening. Open Dent. J. 2019, 13, 436–442. [Google Scholar] [CrossRef]
- Ergün Kunt, G.; Yılmaz, N.; Şen, S.; Dede, D.Ö. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol. Scand. 2011, 69, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.J.; Mena-Serrano, A.; De Andrade, A.M.; Reis, A.; Grande, R.H.; Loguercio, A.D. Immediate bonding to bleached enamel treated with 10% sodium ascorbate gel: A case report with one-year follow-up. Eur. J. Esthet. Dent. 2012, 7, 154–162. [Google Scholar] [PubMed]
- De Paula, E.A.; Kossatz, S.; Fernandes, D.; Loguercio, A.D.; Reis, A. Administration of ascorbic acid to prevent bleaching-induced tooth sensitivity: A randomized tripleblind clinical trial. Oper. Dent. 2014, 39, 128–135. [Google Scholar] [CrossRef]
- Louzada, L.M.; Briso, A.L.F.; Benetti, F.; Vieira, L.B.; Jacinto, R.D.C.; Dezan-Júnior, E.; Cintra, L.T.A. Anti-inflammatory potential of a carvedilol gel in the pulpal tissue of rats after dental bleaching: A histopathological evaluation. J. Investig. Clin. Dent. 2019, 10, e1240. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Shankar, P.; Bannimath, G.; Doddawad, V.; Annapoorna, B. Evaluation of antioxidant property of amla on bond strength and color stability of power bleached teeth: An in vitro study. J. Pharm. Bioallied Sci. 2021, 13, S1244–S1250. [Google Scholar]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. Clin. Ther. 2000, 5, 144–151. [Google Scholar]
- Xie, Q.; Bedran-Russo, A.K.; Wu, C.D. In vitro remineralization effects of grape seed extract on artificial root caries. J. Dent. 2008, 36, 900–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Wang, L.; Zhu, J.; Hu, Z.; Zhang, J. Self-double-emulsifying drug delivery system (SDEDDS): A new way for oral delivery of drugs with high solubility and low permeability. Int. J. Pharm. 2011, 409, 245–251. [Google Scholar] [CrossRef]
- Esquivel-Chirino, C.; Gómez-Landeros, J.C.; Carabantes-Campos, E.P.; Carmona-Ruiz, D.; Valero-Princet, Y.; Márquez-Correa, C.; Morales-González, J.A. The Impact of Oxidative Stress on Dental Implants. Eur. J. Dent. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Triplett, R.G.; Frohberg, U.; Sykaras, N.; Woody, R.D. Implant materials, design, and surface topographies: Their influence on osseointegration of dental implants. J. Long-Term Eff. Med. Implants 2003, 13. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac 2014, 40, 50. [Google Scholar] [CrossRef] [PubMed]
- Crabb, C. History of dental implants. Dent. Nurs. 2006, 2, 398–399. [Google Scholar] [CrossRef]
- Yanez, M.; Blanchette, J.; Jabbarzadeh, E. Modulation of inflammatory response to implanted biomaterials using natural compounds. Curr. Pharm. Des. 2017, 23, 6347–6357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsaryk, R.; Kalbacova, M.; Hempel, U.; Scharnweber, D.; Unger, R.E.; Dieter, P.; Kirkpatrick, C.J.; Peters, K. Response of human endothelial cells to oxidative stress on Ti6Al4V alloy. Biomaterials 2007, 28, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Yoshino, F.; Shoji, H.; Takahashi, S.; Todoki, K.; Shimada, S.; Kuse-Barouch, K. Characterization by electron spin resonance spectroscopy of reactive oxygen species generated by titanium dioxide and hydrogen peroxide. J. Dent. Res. 2005, 84, 178–182. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal nanoparticles released from dental implant surfaces: Potential contribution to chronic inflammation and peri-implant bone loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulhameed, E.A.; Al-Rawi, N.H.; Omar, M.; Khalifa, N.; Samsudin, A.R. Titanium dioxide dental implants surfaces related oxidative stress in bone remodeling: A systematic review. PeerJ 2022, 10, e12951. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [Green Version]
- Jazi, M.M.; Rodsari, H.R.S.P.; Mirmiran, F. Level of oxidative stress markers in peri-implant crevicular fluid and their correlation with clinical parameters. J. Dent. 2015, 12, 340. [Google Scholar]
- Suárez-López del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral. Implants Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, P.A.; Snelling, S.J.; Dakin, S.G.; Milković, L.; Gašparović, A.Č.; Carr, A.J.; Žarković, N. Biocompatibility of implantable materials: An oxidative stress viewpoint. Biomaterials 2016, 109, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food. Drug. Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Tardy, B.L.; Richardson, J.J.; Nithipipat, V.; Kempe, K.; Guo, J.; Cho, K.L.; Rahim, M.A.; Ejima, H.; Caruso, F. Protein adsorption and coordination-based end-tethering of functional polymers on metal-phenolic network films. Biomacromolecules 2019, 20, 1421–1428. [Google Scholar] [CrossRef]
- Harmankaya, N.; Igawa, K.; Stenlund, P.; Palmquist, A.; Tengvall, P. Healing of complement activating Ti implants compared with non-activating Ti in rat tibia. Acta Biomaterialia 2012, 8, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Källtorp, M.; Askendal, A.; Thomsen, P.; Tengvall, P. Inflammatory cell recruitment, distribution, and chemiluminescence response at IgG precoated- and thiol functionalized gold surfaces. J. Biomed. Mater. Res 1999, 47, 251–259. [Google Scholar] [CrossRef]
- Huang, D.; Lin, Q.; Zhou, Y.; Li, J.; Wei, Y.; Hu, Y.; Lian, X.; Chen, S.; Chen, W. Ag nanoparticles incorporated tannic acid/nanoapatite composite coating on Ti implant surfaces for enhancement of antibacterial and antioxidant properties. Surf. Coat. Technol. 2020, 399, 126169. [Google Scholar]
- Maruyama, T.; Tomofuji, T.; Endo, Y.; Irie, K.; Azuma, T.; Ekuni, D.; Tamaki, N.; Yamamoto, T.; Morita, M. Supplementation of green tea catechins in dentifrices suppresses gingival oxidative stress and periodontal inflammation. Arch. Oral Biol. 2011, 56, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Ferrara, C.; Mustarelli, P. Silica–polyethylene glycol hybrids synthesized by sol–gel: Biocompatibility improvement of titanium implants by coating. Mater. Sci. Eng. C 2015, 55, 118–125. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. J. Inflam. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Piccolella, S.; Pacifico, S. Sol–gel synthesis and characterization of SiO2/PCL hybrid materials containing quercetin as new materials for antioxidant implants. Mater. Sci. Eng. C 2016, 58, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Graham, H.D. Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food Chem. 1992, 40, 801–805. [Google Scholar] [CrossRef]
- Boraldi, F.; Annovi, G.; Paolinelli-Devincenzi, C.; Tiozzo, R.; Quaglino, D. The effect of serum withdrawal on the protein profile of quiescent human dermal fibroblasts in primary cell culture. Proteomics 2008, 8, 66–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vroman, L.; Adams, A.L. Findings with the recording ellipsometer suggesting rapid exchange of specific plasma proteins at liquid/solid interfaces. Surf. Sci. 1969, 16, 438–446. [Google Scholar] [CrossRef]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Chow, C.K.; Ibrahim, W.; Wei, Z.; Chan, A.C. Vitamin E regulates mitochondrial hydrogen peroxide generation. Free Radic. Biol. Med. 1999, 27, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Carpentieri, I.; Bracco, P. Post electron-beam irradiation oxidation of orthopaedic Ultra-High Molecular Weight Polyethylene (UHMWPE) stabilized with vitamin E. Polym. Degrad. Stab. 2009, 94, 1542–1547. [Google Scholar] [CrossRef]
- Ozawa, R.; Saita, M.; Sakaue, S.; Okada, R.; Sato, T.; Kawamata, R.; Sakurai, T.; Hamada, N.; Kimotoa, K.; Nagasaki, Y. Redox injectable gel protects osteoblastic function against oxidative stress and suppresses alveolar bone loss in a rat peri-implantitis model. Acta Biomater. 2020, 110, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Zieniewska, I.; Maciejczyk, M.; Zalewska, A. The effect of selected dental Materials used in conservative dentistry, endodontics, surgery, and orthodontics as well as during the periodontal treatment on the redox balance in the oral cavity. Int. J. Mol. Sci. 2020, 21, 9684. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Elkhatib, R. Effect of mercury (Hg) dental amalgam fillings on renal and oxidative stress biomarkers in children. Sci. Total Environ. 2012, 431, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jirau-Colón, H.; González-Parrilla, L.; Martinez-Jiménez, J.; Adam, W.; Jiménez-Velez, B. Rethinking the dental amalgam dilemma: An integrated toxicological approach. Int. J. Environ. 2019, 16, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoldi, A.F.; Coccini, T.; Manzo, L. Neurotoxic and molecular effects of methylmercury in humans. Rev. Environ. Health 2003, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, M.; Fonzi, M.; Sugherini, L.; Fonzi, L.; Gasparoni, A.; Comporti, M.; Pompella, A. Release of mercury from dental amalgam and its influence on salivary antioxidant activity. Sci. Total Environ. 2002, 284, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindh, U.; Hudecek, R.; Danersund, A.; Eriksson, S.; Lindvall, A. Removal of dental amalgam and other metal alloys supported by antioxidant therapy alleviates symptoms and improves quality of life in patients with amalgam-associated ill health. Neuro. Endocrinol. Lett. 2002, 23, 459. [Google Scholar] [PubMed]
- Jan, A.T.; Ali, A.; Haq, Q.M.R. Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity. J. Postgrad. Med. 2011, 57, 72. [Google Scholar]
- Frisk, P.; Danersund, A.; Hudecek, R.; Lindh, U. Changed Clinical Chemistry Pattern in Blood After Removal of Dental Amalgam and other Metal Alloys Supported by Antioxidant Therapy. Biol. Trace Elem. Res. 2007, 120, 163–170. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free. Radical. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Abudu, N.; Miller, J.J.; Attaelmannan, M.; Levinson, S.S. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin. Chim. Acta 2004, 339, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Gallusi, G.; Libonati, A.; Piro, M.; Taranto, V.D.; Montemurro, E.; Campanella, V. Is Dental amalgam a higher risk factor rather than resin-based restorations for systemic conditions? A systematic review. Materials 2021, 14, 1980. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhairy, F. Attitudes of dentists and interns in Riyadh to the use of dental amalgam. BMC Res. Notes 2016, 9, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.; Douglass, C.W.; Kim, H.D.; Joshipura, K.J.; Park, M.C.; Rimm, E.B.; Carino, M.J.; Garcia, R.I.; Morris, J.S.; Willett, W.C. The relationship between amalgam restorations and mercury levels in male dentists and nondental health professionals. J. Public Health Dent. 2007, 63, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, J.; Murray, C.; Schwass, D.; Brosnan, M.; Brunton, P.; Lyons, K.; Thomson, W. The dental amalgam phasedown in New Zealand: A 20-year trend. Oper. Dent. 2020, 45, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 2013, 34, 4555–4563. [Google Scholar] [CrossRef]
- Klapdohr, S.; Moszner, N. New inorganic components for dental filling composites. Monatsh. Chem. 2005, 136, 21–45. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Lavigueur, C.; Zhu, X.X. Recent advances in the development of dental composite resins. RSC Adv. 2012, 2, 59–63. [Google Scholar] [CrossRef]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Mantellini, M.G.; Botero, T.M.; Yaman, P.; Dennison, J.B.; Hanks, C.T.; Nör, J.E. Adhesive resin induces apoptosis and cell-cycle arrest of pulp cells. J. Dent. Res. 2003, 82, 592–596. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M. Biocompatibility of amalgam vs composite–A review. Oral Health 2022, 2022, 149–156. [Google Scholar]
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061. [Google Scholar] [CrossRef] [Green Version]
- Wattamwar, P.P.; Biswal, D.; Cochran, D.B.; Lyvers, A.C.; Eitel, R.E.; Anderson, K.W.; Hilt, J.Z.; Dziubla, T.D. Synthesis and characterization of poly (antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012, 8, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Yamada, M.; Kojima, N.; Ogawa, T. N-Acetyl cysteine prevents suppression of oral fibroblast function on poly(methylmethacrylate) resin. Acta Biomater. 2009, 5, 391–398. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Zingg, J.M.; Azzi, A. The 80th anniversary of vitamin E: Beyond its antioxidant properties. Biol. Chem. 2002, 383, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Newton, A.M.J.; Kaur, B.; Indana, V.L.; Rajesh, K.S. Chronotherapeutic drug delivery of pectin vs. guar gum, xanthan gum controlled release colon targeted directly compressed propranolol Hcl matrix tablets. SAJ Pharm. Pharmacol. 2014, 1, 1–12. [Google Scholar]
- Wilson, A.D.; Kent, B.E. The glass-ionomer cement, a new translucent dental filling material. J. Appl. Chem. 1971, 21, 313. [Google Scholar] [CrossRef]
- Lohbauer, U. Dental glass ionomer cements as permanent filling materials? —Properties, limitations future trends. Materials 2009, 3, 76–96. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kang, S. Current aspects and prospects of glass ionomer cements for clinical dentistry. Yeungnam Univ. J. Med. 2020, 37, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Watson, T.F. Resin-modified glass ionomer materials. A status report for the American Journal of Dentistry. Am. J. Dent. 1995, 8, 59–67. [Google Scholar] [PubMed]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.C.; Magalhães, A.P.R.; Pires, W.C.; Pereira, F.C.; Silveira-Lacerda, E.P.; Carrião, M.S.; Bakuzis, A.F.; Souza-Costa, C.A.; Lopes, L.G.; Estrela, C. Cytotoxicity of glass ionomer cements containing silver nanoparticles. J. Clin. Exp. Dent. 2015, 7, e622. [Google Scholar] [CrossRef] [PubMed]





| Antioxidants | Mechanism of Action | Selected Delivery Form | Ref. |
|---|---|---|---|
| Vitamin C |  | Gel assembled from nanoparticles. | [12,13] |
| Vitamin E |  | Responsive intelligent hydrogel. | [14,15] |
| Quercetin |  | Quercetin-loaded nanoemulsion (QNE), polymeric nanoparticles, liposomes. | [16,17] |
| Oligomeric proanthocyanin complexes (OPCs) |  | Solid, self-double emulsified drug delivery system (SDEDDS). | [18,19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, F.; Huang, H.; Wang, M.; Rong, W.; Wang, J. Applications of Antioxidants in Dental Procedures. Antioxidants 2022, 11, 2492. https://doi.org/10.3390/antiox11122492
Qi F, Huang H, Wang M, Rong W, Wang J. Applications of Antioxidants in Dental Procedures. Antioxidants. 2022; 11(12):2492. https://doi.org/10.3390/antiox11122492
Chicago/Turabian StyleQi, Fan, Haofei Huang, Ming Wang, Weifeng Rong, and Jing Wang. 2022. "Applications of Antioxidants in Dental Procedures" Antioxidants 11, no. 12: 2492. https://doi.org/10.3390/antiox11122492