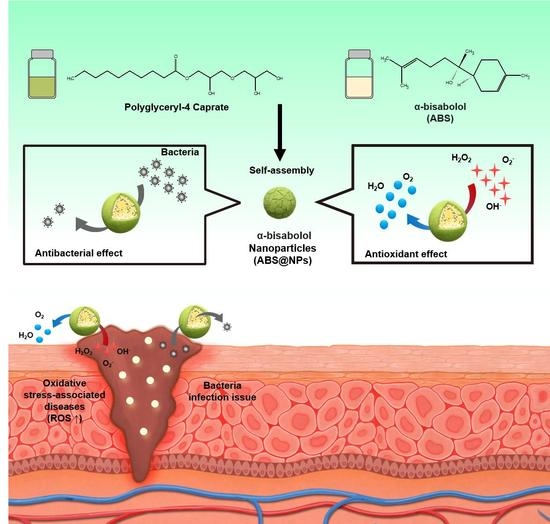
Facile Fabrication of α-Bisabolol Nanoparticles with Improved Antioxidant and Antibacterial Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ABS@NPs
2.3. Methylene Blue Staining of ABS@NPs
2.4. Assessment of the Stability of ABS@NPs
2.5. In Situ Antioxidant Activity of ABS@NPs by the DPPH Assay
2.6. In Vitro Antioxidant Activity of ABS@NPs
2.7. Antibacterial Activity of ABS@NPs
2.8. In Vitro Cytotoxicity of ABS@NPs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ABS@NPs
3.2. Long-Term Stability of The ABS@NPs
3.3. In Situ DPPH Radical-Scavenging Activity and In Vitro Antioxidant Activity of the ABS @NPs
3.4. Antibacterial Activity of ABS@NPs
3.5. In Vitro Cytotoxicity Activity of ABS @NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Dubey, V.S.; Bhalla, R.; Luthra, R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003, 28, 637–646. [Google Scholar] [CrossRef]
- Joshi, S.; Chanotiya, C.S.; Agarwal, G.; Prakash, O.; Pant, A.K.; Mathela, C.S. Terpenoid Compositions, and Antioxidant and Antimicrobial Properties of the Rhizome Essential Oils of Different Hedychium Species. Chem. Biodivers. 2008, 5, 299–309. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yu, S.; Kim, J.; Baek, K.; Lee, Y.R.; Lee, H.S.; Choi, W.I.; Sung, D. Facile Solvent-Free Preparation of Antioxidant Idebenone-Loaded Nanoparticles for Efficient Wound Healing. Pharmaceutics 2022, 14, 521. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Kim, J.; Kim, W.J. Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges. Adv. Sci. 2017, 4, 1600124. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef]
- Choi, S.-I.; Lee, J.S.; Lee, S.; Lee, H.J.; Kim, B.-J.; Yeo, J.; Jung, T.-D.; Cho, B.-Y.; Choi, S.-H.; Lee, J.-H. Antioxidant and anti-aging effects of extracts from leaves of Castanea crenata Siebold & Zucc. in human dermal fibroblast. J. Food Hyg. Saf. 2017, 32, 243–248. [Google Scholar]
- Liu, Y.e.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An epigenetic role of mitochondria in cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, J.; Roman, R.J.; Fan, F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? GeroScience 2022, 44, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Nagasaki, Y. Reactive oxygen species-scavenging nanomedicines for the treatment of oxidative stress injuries. Adv. Healthc. Mater. 2014, 3, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria. Sci. Rep. 2016, 6, 18877. [Google Scholar] [CrossRef] [Green Version]
- Hillyer, C.D.; Josephson, C.D.; Blajchman, M.A.; Vostal, J.G.; Epstein, J.S.; Goodman, J.L. Bacterial contamination of blood components: Risks, strategies, and regulation: Joint ASH and AABB educational session in transfusion medicine. Hematol. Am. Soc. Hematol. Educ. Program 2003, 2003, 575–589. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.A. Food Contamination: Major Challenges of the Future. Foods 2016, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, L. Molecular diagnosis of microbial contamination in cosmetic and pharmaceutical products: A review. J. AOAC Int. 2001, 84, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Y. Potential impacts of silver nanoparticles on bacteria in the aquatic environment. J. Environ. Manag. 2017, 191, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Hughes, O.B.; Maderal, A.D.; Tosti, A. Preservative Sensitization—Safety With and Safety Without. Curr. Treat. Options Allergy 2016, 3, 345–358. [Google Scholar] [CrossRef]
- Magos, L. Review on the toxicity of ethylmercury including its presence as a preservative in biological and pharmaceutical products. J. Appl. Toxicol. 2001, 21, 1–5. [Google Scholar] [CrossRef]
- Herman, A. Antimicrobial Ingredients as Preservative Booster and Components of Self-Preserving Cosmetic Products. Curr. Microbiol. 2019, 76, 744–754. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondi, M.; Lauková, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural Preservatives to Improve Food Quality and Safety. J. Food Qual. 2017, 2017, 1090932. [Google Scholar] [CrossRef] [Green Version]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, J.G.B.; Jornada, D.S.; Silva, F.K.; Freitas, A.L.; Fuentefria, A.M.; Pohlmann, A.R.; Guterres, S.S. Triclosan resistance reversion by encapsulation in chitosan-coated-nanocapsule containing alpha-bisabolol as core: Development of wound dressing. Int. J. Nanomed. 2017, 12, 7855–7868. [Google Scholar] [CrossRef] [Green Version]
- Marongiu, L.; Donini, M.; Bovi, M.; Perduca, M.; Vivian, F.; Romeo, A.; Mariotto, S.; Monaco, H.L.; Dusi, S. The inclusion into PLGA nanoparticles enables α-bisabolol to efficiently inhibit the human dendritic cell pro-inflammatory activity. J. Nanopart. Res. 2014, 16, 2554. [Google Scholar] [CrossRef]
- Terroso, T.F.; Condotta, K.B.; da Fonseca, F.N.; Jornada, D.S.; Ferreira, G.O.; Ellwanger, J.H.; Schmidt, J.A.; Pohlmann, A.R.; Guterres, S.S. In vivo prophylactic gastroprotection using α-bisabolol encapsulated in lipid-core nanocapsules and in cocoa-theospheres. J. Drug Deliv. Sci. Technol. 2016, 36, 99–109. [Google Scholar] [CrossRef]
- Sathya, S.; Shanmuganathan, B.; Devi, K.P. Deciphering the anti-apoptotic potential of alpha-bisabolol loaded solid lipid nanoparticles against Abeta induced neurotoxicity in Neuro-2a cells. Colloids Sur. B Biointerfaces 2020, 190, 110948. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of alpha-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.S.; Woo, J.; Ahn, S.; Lee, E.; Il Choi, W.; Sung, D. Reactive oxygen species (ROS)-responsive ferrocene-polymer-based nanoparticles for controlled release of drugs. J. Mater. Chem. B 2020, 8, 1906–1913. [Google Scholar] [CrossRef]
- Sao Pedro, A.; Detoni, C.; Ferreira, D.; Cabral-Albuquerque, E.; Sarmento, B. Validation of a high-performance liquid chromatography method for the determination of (−)-alpha-bisabolol from particulate systems. Biomed. Chromatogr. 2009, 23, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Woo, J.; Choi, W.I.; Lee, J.H.; Hong, J.; Sung, D. alpha-Tocopherol-loaded reactive oxygen species-scavenging ferrocene nanocapsules with high antioxidant efficacy for wound healing. Int. J. Pharm. 2021, 596, 120205. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, A.P.L.; Pacheco de Oliveira, M.T.; de Souza, E.T.; de Sa Coutinho, D.; Ciambarella, B.T.; Gomes, C.R.; Terroso, T.; Guterres, S.S.; Pohlmann, A.R.; Silva, P.M.; et al. alpha-bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice. Int. J. Nanomed. 2017, 12, 4479–4491. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour, M.; Mobini-Dehkordi, M.; Saffar, B.; Teimori, H. Antiproliferative effects of Matricaria chamomilla on Saccharomyces cerevisiae. J. HerbMed Pharmacol. 2013, 2, 49–51. [Google Scholar]
- Kim, K.W.; Thomas, R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.; Lee, J.S.; Sung, D.; Lim, J.-M.; Choi, W.I. <p>Potential Antioxidant and Wound Healing Effect of Nano-Liposol with High Loading Amount of Astaxanthin. Int. J. Nanomed. 2020, 15, 9231–9240. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; Freitas, T.S.; Cruz, R.P.D.; Costa, M.D.S.; Pereira, R.L.S.; Quintans-Junior, L.J.; Andrade, T.A.; Menezes, P.D.P.; Sousa, B.M.H.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of alpha-bisabolol, beta-cyclodextrin and alpha-bisabolol/beta-cyclodextrin complex. Biomed. Pharm. 2017, 92, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Lee, H.S.; Sung, D.; Choi, W.I. A Novel Polyvinylpyrrolidone-Stabilized Illite Microparticle with Enhanced Antioxidant and Antibacterial Effect. Polymers 2021, 13, 4275. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Shanmuganathan, B.; Manirathinam, G.; Ruckmani, K.; Devi, K.P. α-Bisabolol loaded solid lipid nanoparticles attenuates Aβ aggregation and protects Neuro-2a cells from Aβ induced neurotoxicity. J. Mol. Liq. 2018, 264, 431–441. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutierrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E. Chemical Constituents, In Vitro Antioxidant Activity and In Silico Study on NADPH Oxidase of Allium sativum L. (Garlic) Essential Oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Yu, S.; Kim, J.; Khaliq, N.U.; Choi, W.I.; Kim, H.; Sung, D. Facile Fabrication of α-Bisabolol Nanoparticles with Improved Antioxidant and Antibacterial Effects. Antioxidants 2023, 12, 207. https://doi.org/10.3390/antiox12010207
Kim S, Yu S, Kim J, Khaliq NU, Choi WI, Kim H, Sung D. Facile Fabrication of α-Bisabolol Nanoparticles with Improved Antioxidant and Antibacterial Effects. Antioxidants. 2023; 12(1):207. https://doi.org/10.3390/antiox12010207
Chicago/Turabian StyleKim, Sangwoo, Sohyeon Yu, Jisu Kim, Nisar Ul Khaliq, Won Il Choi, Hyungjun Kim, and Daekyung Sung. 2023. "Facile Fabrication of α-Bisabolol Nanoparticles with Improved Antioxidant and Antibacterial Effects" Antioxidants 12, no. 1: 207. https://doi.org/10.3390/antiox12010207