β-Lactoglobulin Adsorption Layers at the Water/Air Surface: 4. Impact on the Stability of Foam Films and Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Foams
2.3. Foam Films
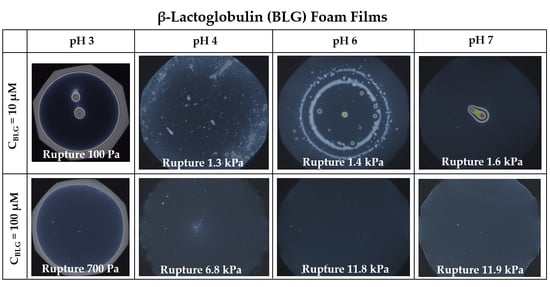
3. Results
3.1. Foam Films
3.2. Foams
4. Discussion
- Group 1: Πcr < . This condition is valid for the foams and films at pH 3 and pH 5 and at CBLG = 10 µM, and can explain the lack of measurable tdev values, where it is possible that the onset of bubble coalescence occurs already in the period of foam formation. These two foams also exhibit the shortest t½ and ttr (in the order of some minutes). For pH 5, this is somehow expected, having in mind that CBLG = 10 µM < Ce and unstable NBF spots emerge in films within seconds (Figure S6 in the Supplementary Materials). For pH 3, the most probable reason is low protein surface excess as suggested by the long induction time in the dynamic surface pressure measurements (Figures S6 and S7 in SI).
- Group 2: < Πcr < . This condition is valid for the foams and films at pH 4, 6, 7 and CBLG = 10 µM, but also for those at pH 3 and CBLG = 100 µM, and results in measurable values for tdev. We should mention here that the time scale of tdev is much shorter than the typical timescale of protein foam film drainage [19,20,61,67,68,69,70,71] (Figure 3 and Figure 6). Therefore, bubble coalescence seemingly takes place for films that are far from equilibrium, which is highly probable for bubbles in the top foam layer of a freshly produced foam [73]. Despite the higher protein concentration at pH 3, foam and film stabilities are still relatively low, and the reason for that is not clear yet [25]. Nevertheless, the good film-foam correlations in groups 1 and 2 demonstrate that single foam film stability is an indicator of foam stability. However, this is not the case for the foams in group 3.
- Group 3: Πcr > . This condition is valid for the foams and films at pH 4–7 and CBLG = 100 µM (as well as for intermediate CBLG at pH 5) and suggests higher resistance of the foam bubbles to coalescence. Indeed, tdev and ttr increase, which agrees with the results in ref. [17]. This fact should be related mainly to the increased protein surface excess at the foam interfaces (as known from adsorption studies [58,60]) that leads to at least two important contributions: (i) stronger immobilization of interfaces, which decelerates the liquid efflux [10,28]; and (ii) stabilization of foam films against rupture [61,71], i.e., impact on the processes of syneresis and coalescence, respectively. The longest tdev at pH 5, 6, 7 are attributable to the low calculated rates of liquid efflux vdev (Equations (S1)) and Figure S4 in the Supplementary Materials) in the initial stage of foam decay (t0–tdev); the longest ttr at pH 6 and pH 7 correspond to the most stable foam films. Concerning the latter, we should note that despite the comparable film stability at pH 6 and pH 7, foam stability diminishes at pH 7.
- Group 4: Πcr > ; pH 5. At CBLG > Ce, Πcr continuously increases with increasing CBLG resulting in increasing foam stability [25]. At CBLG = 100 µM, Πcr for pH 5 and pH 4 are comparable (within the experimental error), but at pH 5 the measured tdev is almost two-fold longer, whereas ttr is shorter. While the latter cannot be straightforwardly explained, the former can be attributed to the much lower vdev (Figure S4 in the Supplementary Materials) as a result of the high surface viscosities either in shear [56] or in dilation [59] (Figure S8 in the Supplementary Materials) as measured at early times of adsorption, as well as from a cork effect in the Plateau borders [24], and entrapment of BLG aggregates in the films (Figure 5). Hence, the foam at pH 5 drains slower than that at pH 4, but the rate of bubble coalescence is higher and a possible explanation for that should be due to the influence of aggregates. It has been shown that foams obtained from mixed dispersions of native and aggregated BLG can be either less or more stable than foams from pure native BLG solutions, and the direction of change of foam stability depends on both the aggregate size and the ratio of native/aggregated entities [24].
5. Concluding Comments and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vignes-Adler, M. Historical Perspectives of Foams. In Foam Films and Foams: Fundamentals and Applications; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 8. [Google Scholar]
- Gochev, G.; Ulaganathan, V.; Miller, R. Foams. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2016; pp. 1–31. [Google Scholar]
- Bikerman, J.J. Foams; Springer: New York, NY, USA, 1973. [Google Scholar]
- Exerowa, D.; Kruglyakov, P.M. Foam and Foam Films; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Cantat, I.; Cohen-Addad, S.; Elias, F.; Graner, F.; Höhler, R.; Pitois, O.; Rouyer, F.; Saint-Jalmes, A.; Cox, S. Foams: Structure and Dynamics; OUP: Oxford, UK, 2013. [Google Scholar]
- Pugh, R. Bubble and Foam Chemistry; CUP: Cambridge, UK, 2016. [Google Scholar]
- Langevin, D. Foam stabilization Mechanisms. In Foam Films and Foams: Fundamentals and Applications; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 11. [Google Scholar]
- Schmidt, C.G.; Gunez, D.Z.; Gehin-Delval, C.; Leser, M.E. Protein-Stabilized Foams. In Foam Films and Foams: Fundamentals and, Applications; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 14. [Google Scholar]
- Murray, B.S. Stabilization of bubbles and foams. Curr. Opin. Colloid Interface Sci. 2007, 12, 232–241. [Google Scholar] [CrossRef]
- Malysa, K.; Lunkenheimer, K. Foams under dynamic conditions. Curr. Opin. Colloid Interface Sci. 2008, 13, 150–162. [Google Scholar] [CrossRef]
- Langevin, D. Aqueous foams: A field of investigation at the frontier between chemistry and physics. Chem. Phys. Chem. 2008, 9, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, P.A.; Gruppen, H. New views on foams from protein solutions. Curr. Opin. Colloid Interface Sci. 2010, 15, 365–373. [Google Scholar] [CrossRef]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Narsimhan, G.X.; Xiang, N. Role of Proteins on Formation, Drainage, and Stability of Liquid Food Foams. Ann. Rev. Food Sci. Technol. 2018, 25, 45–63. [Google Scholar] [CrossRef]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Malysa, K.; Winsel, K.; Geggel, K.; Siegel, S. Novel Method and Parameters for Testing and Characterization of Foam Stability. Langmuir 2009, 26, 3883–3888. [Google Scholar] [CrossRef]
- Álvarez Gómez, J.M.R.P.; Rodríguez Patino, J.M. Formulation Engineering of Food Model Foams Containing Diglycerol Esters and β-Lactoglobulin. Ind. Eng. Chem. Res. 2006, 45, 7510–7519. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Khristov, K. Foams and foam films stabilized by CnTAB: Influence of the chain length and of impurities. J. Colloid Interface Sci. 2005, 286, 710–718. [Google Scholar] [CrossRef]
- Saint-Jalmes, A.; Peugeot, M.-L.; Ferraz, H.; Langevin, D. Differences between protein and surfactant foams: Microscopic properties, stability and coarsening. Colloids Surf. A 2005, 263, 219–225. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.L.; Langevin, D. On the Difference between Foams Stabilized by Surfactants and Whole Casein or β-Casein. Comparison of Foams, Foam Films, and Liquid Surfaces Studies. J. Phys. Chem. B 2008, 112, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Alahverdjieva, V.S.; Khristov, K.; Exerowa, D.; Miller, R. Correlation between adsorption isotherms, thin liquid films and foam properties of protein/surfactant mixtures: Lysozyme/C10DMPO and lysozyme/SDS. Colloids Surf. A 2008, 323, 132–138. [Google Scholar] [CrossRef]
- Wierenga, P.A.; van Norél, L.; Basheva, E.S. Reconsidering the importance of interfacial properties in foam stability. Colloids Surf. A 2009, 344, 72–78. [Google Scholar] [CrossRef]
- Zawala, J.; Todorov, R.; Olszewska, A.; Exerowa, D.; Malysa, K. Influence of pH of the BSA solutions on velocity of the rising bubbles and stability of the thin liquid films and foams. Adsorption 2010, 16, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Rullier, B.; Axelos, M.A.V.; Langevin, D.; Novales, B. β-Lactoglobulin aggregates in foam films: Effect of the concentration and size of the protein aggregates. J. Colloid Interface Sci. 2010, 343, 330–337. [Google Scholar] [CrossRef]
- Lech, F.J.; Delahaije, R.J.B.M.; Meinders, M.B.J.; Gruppen, H.; Wierenga, P.A. Identification of critical concentrations determining foam ability and stability of β-lactoglobulin. Food Hydrocoll. 2016, 57, 46–54. [Google Scholar] [CrossRef]
- Lech, F.J. Foam Properties of Proteins, Low Molecular Weight Surfactants and Their Complexes. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Braunschweig, B.; Schulze-Zachau, F.; Nagel, E.; Engelhardt, K.; Stoyanov, S.; Gochev, G.; Khristov, K.; Mileva, E.; Exerowa, D.; Miller, R.; et al. Specific effects of Ca2+ ions and molecular structure of β-lactoglobulin interfacial layers that drive macroscopic foam stability. Soft Matter 2016, 12, 5995–6004. [Google Scholar] [CrossRef] [Green Version]
- Prins, A. Stagnant surface behaviour and its effect on foam and film stability. Colloids Surf. A 1999, 149, 467–473. [Google Scholar] [CrossRef]
- Krasowska, M.; Hristova, E.; Khristov, K.; Malysa, K.; Exerowa, D. Isoelectric state and stability of foam films, bubbles and foams from PEO-PPO-PEO triblock copolymer (P-85). Colloid Polym. Sci. 2006, 284, 475–481. [Google Scholar] [CrossRef]
- Beneventi, D.; Carre, B.; Gandini, A. Role of surfactant structure on surface and foaming properties. Colloids Surf. A 2001, 189, 65–73. [Google Scholar] [CrossRef]
- Martin, A.H.; Grolle, K.; Bos, M.A.; Stuart, M.A.C.; van Vliet, T. Network forming properties of various proteins adsorbed at the air/water interface in relation to foam stability. J. Colloid Interface Sci. 2002, 254, 175–183. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Martín-Rodriguez, A.; Gálvez-Ruiz, M.J.; Miller, R.; Langevin, D.; Cabrerizo-Vílchez, M.A. Foams and emulsions of β-casein examined by interfacial rheology. Colloids Surf. A 2008, 323, 116–122. [Google Scholar] [CrossRef]
- Blijdenstein, T.B.J.; de Groot, P.W.N.; Stoyanov, S.D. On the link between foam coarsening and surface rheology: Why hydrophobins are so different. Soft Matter 2010, 6, 1799–1808. [Google Scholar] [CrossRef]
- Dimitrova, L.M.; Petkov, P.V.; Kralchevsky, P.A.; Stoyanov, S.D.; Pelan, E.G. Production and characterization of stable foams with fine bubbles from solutions of hydrophobin HFBII and its mixtures with other proteins. Colloids Surf. A 2017, 521, 92–104. [Google Scholar] [CrossRef]
- Engelhardt, K.; Lexis, M.; Gochev, G.; Konnerth, C.; Miller, R.; Willenbacher, N.; Peukert, W.; Braunschweig, B. pH Effects on the Molecular Structure of β-Lactoglobulin Modified Air–Water Interfaces and Its Impact on Foam Rheology. Langmuir 2013, 29, 11646–11655. [Google Scholar] [CrossRef] [PubMed]
- Lexis, M.; Willenbacher, N. Yield stress and elasticity of aqueous foams from protein and surfactant solutions – The role of continuous phase viscosity and interfacial properties. Colloids Surf. A 2014, 459, 177–185. [Google Scholar] [CrossRef]
- Lexis, M.; Willenbacher, N. Relating foam and interfacial rheological properties of β-lactoglobulin solutions. Soft Matter 2014, 10, 9626–9636. [Google Scholar] [CrossRef] [Green Version]
- Willenbacher, N.; Lexis, M. Foam Rheology. In Foam Films and Foams: Fundamentals and Applications; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 17. [Google Scholar]
- Wan, Z.; Yang, X.; Sagis, L.M.C. Contribution of Long Fibrils and Peptides to Surface and Foaming Behavior of Soy Protein Fibril System. Langmuir 2016, 32, 8092–8101. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Li, J.; Tang, C.; Li, B. Foams Stabilized by β-Lactoglobulin Amyloid Fibrils: Effect of pH. J. Agric. Food Chem. 2017, 65, 10658–10665. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Xu, Y.; Zhang, K.; Nishinari, K.; Phillips, G.O.; Fang, Y. Comparative study on foaming and emulsifying properties of different beta-lactoglobuline aggregates. Food Funct. 2019, 10, 5922–5930. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, J.; Dechau, J.; Kulozik, U. Multiscale approach to characterize bulk, surface and foaming behavior of casein micelles as a function of alkalinisation. Food Hydrocoll. 2016, 57, 92–102. [Google Scholar] [CrossRef]
- Dombrowski, J.; Johler, F.; Warncke, M.; Kulozik, U. Correlation between bulk characteristics of aggregated β-Lactoglobulin and its interfacial foaming properties. Food Hydrocoll. 2016, 61, 318–328. [Google Scholar] [CrossRef]
- Dombrowski, J.; Gschwendtner, M.; Kulozik, U. Evaluation of structural characteristics determining surface and foaming properties of β-Lactoglobulin aggregates. Colloids Surf. A 2017, 516, 286–295. [Google Scholar] [CrossRef]
- Dombrowski, J.; Gschwendtner, M.; Saalfeld, D.; Kulozik, U. Salt-dependent interaction behavior of β-Lactoglobulin in relation to its surface and foaming properties. Colloids Surf. A 2018, 558, 455–462. [Google Scholar] [CrossRef]
- Qiao, X.; Miller, R.; Schneck, E.; Sun, K. Influence of pH on the surface and foaming properties of aqueous silk fibroin solutions. Soft Matter 2020, 16, 3695–3704. [Google Scholar] [CrossRef]
- Engelhardt, K.; Rumpel, A.; Walter, J.; Dombrowski, J.; Kulozik, U.; Braunschweig, B.; Peukert, W. Protein Adsorption at the Electrified Air−Water Interface: Implications on Foam Stability. Langmuir 2012, 28, 7780–7787. [Google Scholar] [CrossRef]
- Richert, M.E.; García Rey, N.; Braunschweig, B. Charge-Controlled Surface Properties of Native and Fluorophore-Labeled Bovine Serum Albumin at the Air–Water Interface. J. Phys. Chem. B 2018, 122, 10377–10383. [Google Scholar] [CrossRef] [Green Version]
- Richert, M.E.; Gochev, G.; Braunschweig, B. Specific Ion Effects of Trivalent Cations on the Structure and Charging State of β-Lactoglobulin Adsorption Layers. Langmuir 2019, 35, 11299–11307. [Google Scholar] [CrossRef]
- Schnurbus, M.; Kabat, M.; Jarek, E.; Krzan, M.; Warszynski, P.; Braunschweig, B. Spiropyran Sulfonates for Photo- and pH-Responsive Air–Water Interfaces and Aqueous Foam. Langmuir 2020, 36, 6871–6879. [Google Scholar] [CrossRef]
- Honnigfort, C.; Campbell, R.A.; Droste, J.; Ravoo, B.J.; Braunschweig, B. Unexpected monolayer-to-bilayer transition of arylazopyrazole surfactants facilitates superior photo-control of fluid interfaces and colloids. Chem. Sci. 2020, 11, 2085–2092. [Google Scholar] [CrossRef] [Green Version]
- Khristov, K. Role of Foam Films in Foam Stability. In Foam Films and Foams: Fundamentals and Applications; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 12. [Google Scholar]
- Khristov, K.; Exerowa, D.; Minkov, G. Critical capillary pressure for destruction of single foam films and foam: Effect of foam film size. Colloids Surf. A 2002, 210, 159–166. [Google Scholar] [CrossRef]
- Dukhin, S.S.; Kovalchuk, V.I.; Aksenenko, E.V.; Miller, R. Surfactant accumulation within the top foam layer due to rupture of external foam films. Adv. Colloid Interface Sci. 2008, 137, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, L.; Schmitt, C. Globular plant protein aggregates for stabilization of food foams and emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar] [CrossRef]
- Celebioglu, H.Y.; Kmiecik-Palczewska, J.; Lee, S.; Chronakis, I.S. Interfacial shear rheology of β-lactoglobulin—Bovine submaxillary mucin layers adsorbed at air/water interface. Int. J. Biol. Macromol. 2017, 102, 857–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, J.; Krägel, J.; Gochev, G.; Ulaganathan, V.; Javadi, A.; Makievski, A.; Miller, R. Bubble–bubble interaction in aqueous β-Lactoglobulin solutions. Food Hydrocoll. 2014, 34, 15–21. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Retzlaff, I.; Won, J.; Gochev, G.; Gehin-Delval, C.; Leser, M.; Noskov, B.A.; Miller, R. β-Lactoglobulin adsorption layers at the water/air surface: 1. Adsorption kinetics and surface pressure isotherm: Effect of pH and ionic strength. Colloids Surf. A 2017, 519, 153–160. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Retzlaff, I.; Won, J.; Gochev, G.; Gunes, D.; Gehin-Delval, C.; Leser, M.; Noskov, B.A.; Miller, R. β-Lactoglobulin adsorption layers at the water/air surface: 2. Dilational rheology: Effect of pH and ionic strength. Colloids Surf. A 2017, 521, 167–176. [Google Scholar] [CrossRef]
- Gochev, G.; Scoppola, E.; Campbell, R.; Noskov, B.A.; Miller, R.; Schneck, E. β-Lactoglobulin Adsorption Layers at the Water/Air Surface: 3. Neutron Reflectivity Study on the Effect of pH. J. Phys. Chem. B 2019, 123, 10877–10889. [Google Scholar] [CrossRef]
- Gochev, G.; Retzlaff, I.; Exerowa, D.; Miller, R. Electrostatic stabilization of foam films from β-lactoglobulin solutions. Colloid Surf. A 2014, 460, 272–279. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Gochev, G.; Gehin-Delval, C.; Leser, M.; Gunes, D.; Miller, R. Effect of pH and electrolyte concentration on rising air bubbles in β-lactoglobulin solutions. Colloids Surf. A 2016, 505, 165–170. [Google Scholar] [CrossRef]
- Toro-Sierra, J.; Tolkach, A.; Kulozik, U. Fractionation of α-lactalbumin and β-lactoglobulin from whey protein isolate using selective thermal aggregation, an optimized membrane separation procedure and resolubilization techniques at pilot plant scale. Food Bioprocess Technol. 2013, 6, 1032–1043. [Google Scholar] [CrossRef]
- Exerowa, D.; Kashchiev, D.; Platikanov, D. Stability and Permeability of Amphiphile Bilayers. Adv. Colloid Interface Sci. 1992, 40, 201–256. [Google Scholar] [CrossRef]
- Platikanov, D.; Exerowa, D. Fundamentals of Foam Films. In Foam Films and Foams: Fundamentals and Applications; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 3. [Google Scholar]
- Garciá Rey, N.; Weißenborn, E.; Schulze-Zachau, F.; Gochev, G.; Braunschweig, B. Quantifying Double-Layer Potentials at Liquid-Gas Interfaces from Vibrational Sum-Frequency Generation. J. Phys. Chem. C 2019, 123, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yampolskaya, G.; Platikanov, D. Proteins at fluid interfaces: Adsorption layers and thin liquid films. Adv. Colloid Interface Sci. 2006, 128–130, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.C.; Coke, M.; Mackie, A.R.; Pinder, A.C.; Wilson, D.R. Molecular diffusion and thickness measurements of protein-stabilized thin liquid films. J. Colloid Interface Sci. 1990, 138, 207–219. [Google Scholar] [CrossRef]
- Cascão Pereira, L.G.; Johansson, C.; Radke, C.J.; Blanch, H.W. Surface Forces and Drainage Kinetics of Protein-Stabilized Aqueous Films. Langmuir 2003, 19, 7503–7513. [Google Scholar] [CrossRef]
- Blomqvist, B.R.; Ridout, M.J.; Mackie, A.R.; Claesson, P.M.; Wilde, P. Disruption of viscoelastic β-lactoglobulin surface layers at the air-water interface by nonionic polymeric surfactants. Langmuir 2004, 20, 10150–10158. [Google Scholar] [CrossRef]
- Petkova, V.S.; Sultanem, C.; Nedyalkov, M.; Benattar, J.-J.; Leser, M.E.; Schmitt, C. Structure of a Freestanding Film of β-Lactoglobulin. Langmuir 2003, 19, 6942–6949. [Google Scholar] [CrossRef]
- Zawala, J.; Kosior, D.; Malysa, K. Formation and influence of the dynamic adsorption layer on kinetics of the rising bubble collisions with solution/gas and solution/solid interfaces. Adv. Colloid Interface Sci. 2015, 222, 765–778. [Google Scholar] [CrossRef]
- Malysa, K.; Cohen, R.; Exerowa, D.; Pomianowski, A. Steady-State Foaming and the Properties of Thin Liquid Films from Aqueous Alcohol Solutions. J. Colloid Interface Sci. 1981, 80, 1–6. [Google Scholar] [CrossRef]
- Uhlig, M.; Löhmann, O.; Vargas Ruiz, S.; Varga, I.; von Klitzing, R.; Campbell, R.A. New structural approach to rationalize the foam film stability of oppositely charged polyelectrolyte/surfactant mixtures. Chem. Commun. 2020, 56, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Verheul, M.; Pedersen, J.S.; Roefs, S.P.F.M.; de Kruif, K.G. Association behavior of native β-lactoglobulin. Biopolymers 1999, 49, 11–20. [Google Scholar] [CrossRef]
- Taulier, N.; Chalikian, T.V. Characterization of pH-induced transitions of β-lactoglobulin: Ultrasonic, densimetric, and spectroscopic studies. J. Mol. Biol. 2001, 314, 873–889. [Google Scholar] [CrossRef] [Green Version]
- Aymard, P.; Durand, D.; Nicolai, T. The effect of temperature and ionic strength on the dimerisation of β-lactoglobulin. Int. J. Biol. Macromol. 1996, 19, 213–221. [Google Scholar] [CrossRef]
- Sakai, K.; Sakurai, K.; Sakai, M.; Hoshino, M.; Goto, Y. Conformation and stability of thiol-modified bovine β-lactoglobulin. Prot. Sci. 2000, 9, 1719–1729. [Google Scholar]
- Sakurai, K.; Oobatake, M.; Goto, Y. Salt-dependent monomer-dimer equilibrium of bovine β-lactoglobulin at pH 3. Prot. Sci. 2001, 10, 2325–2335. [Google Scholar] [CrossRef]
- Mercadante, D.; Melton, L.D.; Norris, G.E.; Dobson, R.C.J.; Jameson, G.B. Bovine β-lactoglobulin is dimeric under imitative physiological conditions: Dissociation equilibrium and rate constants over the pH range of 2.5–7.5. Biophys. J. 2012, 103, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basheva, E.S.; Kralchevsky, P.A.; Danov, K.D.; Stoyanov, S.D.; Blijdenstein, T.B.J.; Pelan, E.G.; Lips, A. Self-Assembled Bilayers from the Protein HFBII Hydrophobin: Nature of the Adhesion Energy. Langmuir 2011, 27, 4481–4488. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gochev, G.G.; Ulaganathan, V.; Retzlaff, I.; Gehin-Delval, C.; Gunes, D.Z.; Leser, M.; Kulozik, U.; Miller, R.; Braunschweig, B. β-Lactoglobulin Adsorption Layers at the Water/Air Surface: 4. Impact on the Stability of Foam Films and Foams. Minerals 2020, 10, 636. https://doi.org/10.3390/min10070636
Gochev GG, Ulaganathan V, Retzlaff I, Gehin-Delval C, Gunes DZ, Leser M, Kulozik U, Miller R, Braunschweig B. β-Lactoglobulin Adsorption Layers at the Water/Air Surface: 4. Impact on the Stability of Foam Films and Foams. Minerals. 2020; 10(7):636. https://doi.org/10.3390/min10070636
Chicago/Turabian StyleGochev, Georgi G., Vamseekrishna Ulaganathan, Inga Retzlaff, Cécile Gehin-Delval, Deniz Z. Gunes, Martin Leser, Ulrich Kulozik, Reinhard Miller, and Björn Braunschweig. 2020. "β-Lactoglobulin Adsorption Layers at the Water/Air Surface: 4. Impact on the Stability of Foam Films and Foams" Minerals 10, no. 7: 636. https://doi.org/10.3390/min10070636