Fabrication of Solvent-Free PCL/β-TCP Composite Fiber for 3D Printing: Physiochemical and Biological Investigation
Abstract
:1. Introduction
2. Materials and Methods
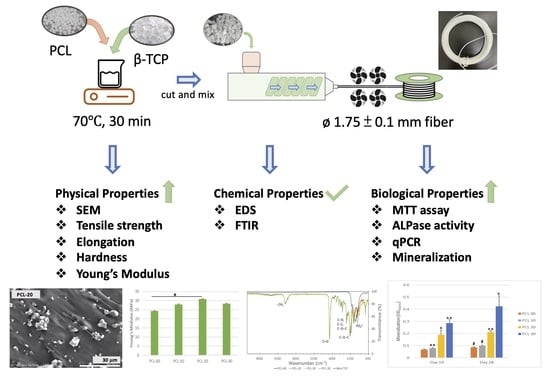
2.1. Fabrication of PCL/β-TCP Fiber and 3D Scaffold
2.2. PCL/β-TCP Material Mechanical Characterization
2.2.1. Scanning Electron Microscopy and Energy Dispersive Spectrometry (EDS) Analysis
2.2.2. Fourier Transform Infrared Spectroscopy
2.2.3. PCL/β-TCP Filaments Mechanical Testing
2.2.4. Wettability Test
2.3. PCL/β-TCP Material Biological Characterization
2.3.1. Preparation of Medium Extraction from PCL/β-TCP Fiber
2.3.2. Cell Viability and Biocompatibility
2.3.3. Alkaline Phosphatase Activity
2.3.4. Real-Time Polymerase Chain Reaction (qPCR)
2.3.5. Mineralization Assay
2.3.6. Statistical Analysis
3. Results
3.1. Morphology and Chemical Properties of PCL/β-TCP Fiber
3.2. Physical and Mechanical Evaluation of PCL/β-TCP Fiber
3.3. Inductive Effect of PCL/β-TCP Composite on MG63
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, N.; Nguyen, T.; Kavanagh, N.M.; Cho, Y.D.; Kavanagh, N.M.; Ghassib, I.; Giannobile, W.V. Personalized Scaffolding Technologies for Alveolar Bone Regenerative Medicine. Orthod. Craniofac. Res. 2019, 22 (Suppl. S1), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, X.; Meng, H.; Sun, B.; Chen, P.; Liu, X.; Zhang, K.; Yang, X.; Peng, J.; Lu, S. 3D Printing Porous Ceramic Scaffold for Bone Tissue Engineering: A Review. Biomater. Sci. 2017, 5, 1690–1698. [Google Scholar]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Lala, S.D. Scaffold Development Using Biomaterials: A Review. Mater. Today Proc. 2018, 5, 12909–12919. [Google Scholar] [CrossRef]
- Özcan, M.; Hotza, D.; Fredel, M.C.; Cruz, A.; Volpato, C.A.M. Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry. J. Compos. Sci. 2021, 5, 78. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, M.; Backman, L.J.; Chen, J. Effects of Zinc, Magnesium, and Iron Ions on Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 2321–2335. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Joint 2016, 1, 27. [Google Scholar] [CrossRef]
- Raffaele, C.; Adriana, D.L.; Orsolina, P.; Carlo, R.; Francesco, R.; Anna, D.S.; Anna, C.; Gianfranco, P. Chapter 7: Biodegradable polymers for dental tissue engineering and regeneration. In Biodegradable Polymers: Recent Developments and New Perspectives; Geraldine, R., Ed.; IAPC: Zagreb, Croatia, 2017; pp. 239–279. [Google Scholar]
- Galli, M.; Yao, Y.; Giannobile, W.V.; Wang, H.L. Current and future trends in periodontal tissue engineering and bone regeneration. Plast. Aesthet. Res. 2021, 8, 3. [Google Scholar] [CrossRef]
- Navaei, T.; Milan, P.B.; Davari, H.R.; Samadikuchaksaraei, A.; Mozafari, M. Chapter 15 Nanoengineered biomaterials for diaphragm regeneration. In Nanoengineered Biomaterials for Regenerative Medicine, 1st ed.; Masoud, M., Jayakumar, R., David, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–362. [Google Scholar]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Klaas, D. Clinical applications of calcium phosphate biomaterials: A review. Ceramic. Int. 1993, 19, 363–366. [Google Scholar]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Kasin, N.H.A. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Chen, S.-M.; Lin, S.-F.; Liang, H.-C.; Wu, C.-C. Clinical Efficacy of Polycaprolactone β-Calcium Triphosphate Composite for Osteoconduction in Rabbit Bone Defect Model. Polymers 2021, 13, 2552. [Google Scholar] [CrossRef]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA filament as Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Matsuno, T.; Omata, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Alveolar bone tissue engineering using composite scaffolds for drug delivery. Jpn. Dent. Sci. Rev. 2010, 46, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Zolfagharian, A.; Bodaghi, M.; Baghani, M. A New Strategy for Achieving Shape Memory Effects in 4D Printed Two-Layer Composite Structures. Polymers 2022, 14, 5446. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Soltanmohammadi, K.; Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 4D printing of PET-G via FDM including tailormade excess third shape. MFGLET 2022, 33, 1–4. [Google Scholar] [CrossRef]
- Hamza, H. Dental 4D Printing: An Innovative approach. Innovations 2018, 1, e17. [Google Scholar]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R.; Kumar, L. Significance of 4D printing for dentistry: Materials, process, and potentials. JOBCR 2022, 12, 388–395. [Google Scholar] [CrossRef]
- Joydip, K.; Falguni, P.; Young, H.J.; Cho, D.W. Chapter 2: Biomaterials for Biofabrication of 3D Tissue Scaffolds. In Biofabrication; Gabor, F., Sun, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 23–46. [Google Scholar]
- Cho, Y.S.; Choi, S.; Lee, S.-H.; Kim, K.K.; Cho, Y.-S. Assessments of polycaprolactone/hydroxyapatite composite scaffold with enhanced biomimetic mineralization by exposure to hydroxyapatite via a 3D-printing system and alkaline erosion. Eur. Polym. J. 2019, 113, 340–348. [Google Scholar] [CrossRef]
- Pae, H.-C.; Kang, J.-H.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Jung, U.-W.; Kim, B.-H.; Choi, S.-H. 3D-printed polycaprolactone scaffold mixed with β-tricalcium phosphate as a bone regenerative material in rabbit calvarial defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1254–1263. [Google Scholar] [CrossRef]
- Shim, J.-H.; Jeong, J.-H.; Won, J.-Y.; Bae, J.-H.; Ahn, G.; Jeon, H.; Yun, W.-S.; Bae, E.-B.; Choi, J.-W.; Lee, S.-H. Porosity effect of 3D-printed polycaprolactone membranes on calvarial defect model for guided bone regeneration. Biomed. Mater. 2017, 13, 015014. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Soltanmohammadi, K.; Aberoumand, M.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. Development of Pure Poly Vinyl Chloride (PVC) with Excellent 3D Printability and Macro- and Micro-Structural Properties. Macromol. Mater. Eng. 2022, 2200568. [Google Scholar] [CrossRef]
- Osathanon, T.; Chanjavanakul, P.; Kongdecha, P.; Clayhan, P.; Huynh, N.C.N. Chapter 8 Polycaprolactone-based biomaterials for guided tissue regeneration membrane. In Periodontitis—A Useful Reference, 15th ed.; Pachiappan, A., Ed.; IntechOpen: London, UK, 2017; pp. 171–188. [Google Scholar]
- Zhao, J.; Watanabe, T.; Bhawal, U.K.; Kubota, E.; Abiko, Y. Transcriptome analysis of β–TCP implanted in dog mandible. Bone 2011, 48, 864–877. [Google Scholar] [CrossRef]
- Horowitz, R.A.; Leventis, M.D.; Rohrer, M.D.; Prasad, H.S. Bone grafting: History, rationale, and selection of materials and techniques. Compend. Contin. Educ. Dent. 2014, 35, 1–6. [Google Scholar] [PubMed]
- Chawla, K.; Lamba, A.K.; Faraz, F.; Tandon, S. Evaluation of β-tricalcium phosphate in human infrabony periodontal osseous defects: A clinical study. Quintessence Int. 2011, 42, 291–300. [Google Scholar] [PubMed]
- Gorla, L.F.; Spin-Neto, R.; Boos, F.B.; Pereira, R.S.; Garcia-Junior, I.R.; Hochuli-Vieira, E. Use of autogenous bone and beta-tricalcium phosphate in maxillary sinus lifting: A prospective, randomized, volumetric computed tomography study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.; Moses, O.; Palti, A.; Ormianer, Z. Long-term results of implants immediately placed into extraction sockets grafted with beta-tricalcium phosphate: A retrospective study. J. Oral Maxillofac. Surg. 2013, 71, e63–e68. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-Printed Polycaprolactone/β-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef] [Green Version]
- Baykan, E.; Koc, A.; Elcin, A.E.; Elcin, Y.M. Evaluation of a biomimetic poly(ε-caprolactone)/β-tricalcium phosphate multispiral scaffold for bone tissue engineering: In vitro and in vivo studies. Biointerphases 2014, 9, 029011. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2008, 90, 906–919. [Google Scholar]
- Roque, R.; Barbosa, G.F.; Guastaldi, A.C. Design and 3D bioprinting of interconnected porous scaffolds for bone regeneration. An additive manufacturing approach. J. Manuf. Process. 2021, 64, 655–663. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D printed PCL/β–TCP cross-scale scaffold with high-precision fiber for providing cell growth and forming bones in the pores. Mater. Sci. Eng. C 2021, 127, 112197. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.C.; Yao, W.L.; Choy, C.S.; Pan, Y.H.; Teng, N.-C.; Chang, W.-J. Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers 2020, 12, 93. [Google Scholar] [CrossRef] [Green Version]
- Francisco, I.; Basílio, Â.; Ribeiro, M.P.; Nunes, C.; Travassos, R.; Marques, F.; Pereira, F.; Paula, A.B.; Carrilho, E.; Marto, C.M.; et al. Three-Dimensional Impression of Biomaterials for Alveolar Graft: Scoping Review. J. Funct. Biomater. 2023, 14, 76. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β–TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Raz, P.; Brosh, T.; Romen, G.; Tal, H. Tensile Properties of Three Selected Collagen Membranes. BioMed Res. Int. 2019, 2019, 5163603. [Google Scholar] [CrossRef]
- Lakatos, É.; Magyar, L.; Bojtár, I. Material Properties of the Mandibular Trabecular Bone. J. Med. Eng. 2014, 2014, 470539. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Kalita, V.I.; Komlev, D.I.; Radiuk, A.A.; Fomin, A.S.; Davidova, G.A.; Fursova, N.K.; Murzakhanov, F.F.; Gafurov, M.R.; Fosca, M.; et al. In Vitro Properties of Manganese-Substituted Tricalcium Phosphate Coatings for Titanium Biomedical Implants Deposited by Arc Plasma. Materials 2020, 13, 4411. [Google Scholar] [CrossRef]
- Morgan, R.A. Water contact angle is not a good predictor of biological responses to materials. Biointerphases 2017, 12, 02C201. [Google Scholar]
- Juan, P.-K.; Fan, F.-Y.; Lin, W.-C.; Liao, P.-B.; Huang, C.-F.; Shen, Y.-K.; Ruslin, M.; Lee, C.-H. Bioactivity and Bone Cell Formation with Poly-ε-Caprolactone/Bioceramic 3D Porous Scaffolds. Polymers 2021, 13, 2718. [Google Scholar] [CrossRef]
- Kambur, N.Ö.; Derman, O.; Şen, T.A.; Kinik, E. Osteocalcin. A biochemical marker of bone turnover during puberty. Int. J. Adolesc. Med. Health 2002, 14, 235–244. [Google Scholar]
- Heo, J.S.; Lee, S.G.; Kim, H.O. Distal-less homeobox 5 is a master regulator of the osteogenesis of human mesenchymal stem cells. Inj. J. Mol. Med. 2017, 40, 1486–1494. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-J.; Lee, M.-H.; Wozney, J.M.; Cho, J.-Y.; Ryoo, H.-M. Bone Morphogenetic Protein-2-induced Alkaline Phosphatase Expression Is Stimulated by Dlx5 and Repressed by Msx2. JBC 2004, 279, 50773–50780. [Google Scholar] [CrossRef] [Green Version]
- Hojo, H.; Ohba, S. Sp7 Action in the Skeleton: Its Mode of Action, Functions, and Relevance to Skeletal Diseases. Int. J. Mol. Sci. 2022, 23, 5647. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Symbol | β-TCP/Total Weight (wt%) | β-TCP Powder (g) | PCL (g) |
|---|---|---|---|
| PCL-00 | 0 | 0 | 100 |
| PCL-10 | 10 | 10 | 90 |
| PCL-20 | 20 | 30 | 120 |
| PCL-30 | 30 | 45 | 105 |
| C | O | Ca | P | |
|---|---|---|---|---|
| PCL-00 | 64.5% ± 1.8 | 35.5% ± 1.8 | 0% | 0% |
| PCL-10 | 62.2% ± 0.7 | 34.1% ± 2.8 | 2.1% ± 1.4 | 3.4% ± 0.4 |
| PCL-20 | 57.9% ± 2.2 | 32.2% ± 3.5 | 4.7% ± 2.0 | 5.2% ± 3.4 |
| PCL-30 | 56.9% ± 3.1 | 30.2% ± 2.4 | 6.2% ± 2.0 | 6.7% ± 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, S.T.; Lee, W.-F.; Wu, Y.-F.; Salamanca, E.; Aung, L.M.; Chao, Y.-Q.; Tsao, T.-C.; Hseuh, H.-W.; Lee, Y.-H.; Wang, C.-C.; et al. Fabrication of Solvent-Free PCL/β-TCP Composite Fiber for 3D Printing: Physiochemical and Biological Investigation. Polymers 2023, 15, 1391. https://doi.org/10.3390/polym15061391
Ngo ST, Lee W-F, Wu Y-F, Salamanca E, Aung LM, Chao Y-Q, Tsao T-C, Hseuh H-W, Lee Y-H, Wang C-C, et al. Fabrication of Solvent-Free PCL/β-TCP Composite Fiber for 3D Printing: Physiochemical and Biological Investigation. Polymers. 2023; 15(6):1391. https://doi.org/10.3390/polym15061391
Chicago/Turabian StyleNgo, Sin Ting, Wei-Fang Lee, Yi-Fan Wu, Eisner Salamanca, Lwin Moe Aung, Yan-Qiao Chao, Ting-Chia Tsao, Hao-Wen Hseuh, Yi-Huan Lee, Ching-Chiung Wang, and et al. 2023. "Fabrication of Solvent-Free PCL/β-TCP Composite Fiber for 3D Printing: Physiochemical and Biological Investigation" Polymers 15, no. 6: 1391. https://doi.org/10.3390/polym15061391