Achieving High Thermal Conductivity and Satisfactory Insulating Properties of Elastomer Composites by Self-Assembling BN@GO Hybrids
Abstract
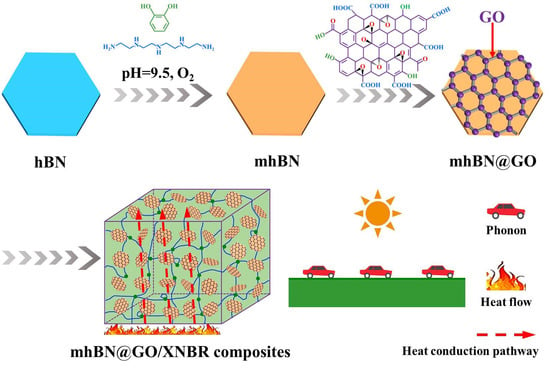
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Process of mhBN@GO/XNBR Composites
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.; Du, G.; Gao, W.; Bai, H. An anisotropically high thermal conductive boron nitride/epoxy composite based on nacre-mimetic 3D network. Adv. Funct. Mater. 2019, 29, 1900412. [Google Scholar] [CrossRef]
- Guo, S.; Zheng, R.; Jiang, J.; Yu, J.; Dai, K.; Yan, C. Enhanced thermal conductivity and retained electrical insulation of heat spreader by incorporating alumina-deposited graphene filler in nano-fibrillated cellulose. Compos. Part B Eng. 2019, 178, 107489. [Google Scholar] [CrossRef]
- Guo, Y.; Lyu, Z.; Yang, X.; Lu, Y.; Ruan, K.; Wu, Y.; Kong, J.; Gu, J. Enhanced thermal conductivities and decreased thermal resistances of functionalized boron nitride/polyimide composites. Compos. Part B Eng. 2019, 164, 732–739. [Google Scholar] [CrossRef]
- Tang, L.; He, M.; Na, X.; Guan, X.; Zhang, R.; Zhang, J.; Gu, J. Functionalized glass fibers cloth/spherical BN filler/epoxy laminated composites with excellent thermal conductivities and electrical insulation properties. Compos. Commun. 2019, 16, 5–10. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Chen, J. Thermal transport in conductive polymer-based materials. Adv. Funct. Mater. 2020, 30, 1904704. [Google Scholar] [CrossRef]
- Niu, H.; Ren, Y.; Guo, H.; Małycha, K.; Orzechowski, K.; Bai, S.-L. Recent progress on thermally conductive and electrical insulating rubber composites: Design, processing and applications. Compos. Commun. 2020, 22, 100430. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Wang, H.; Zhao, F.; Zhang, D.; Chen, Y. Improvement in thermal conductivity of through-plane aligned boron nitride/silicone rubber composites. Mater. Des. 2019, 165, 107580. [Google Scholar] [CrossRef]
- Gan, L.; Dong, M.; Han, Y.; Xiao, Y.; Yang, L.; Huang, J. Connection-improved conductive network of carbon nanotubes in a rubber cross-link network. ACS Appl. Mater. Interfaces 2018, 10, 18213–18219. [Google Scholar] [CrossRef]
- Liu, C.; Wu, W.; Drummer, D.; Shen, W.; Wang, Y.; Schneider, K.; Tomiak, F. ZnO nanowire-decorated Al2O3 hybrids for improving the thermal conductivity of polymer composites. J. Mater. Chem. C 2020, 8, 5380–5388. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, H.; Ruan, K.; Wang, S.; Zhang, Y.; Gu, J. Flexible and insulating silicone rubber composites with sandwich structure for thermal management and electromagnetic interference shielding. Compos. Sci. Technol. 2022, 219, 109253. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Han, Y.; Li, Y.; Ma, T.; Chen, M.; Kong, J.; Zhu, J.; Gu, J. Significant improvement of thermal conductivities for BNNS/PVA composite films via electrospinning followed by hot-pressing technology. Compos. Part B Eng. 2019, 175, 107070. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Liao, M.; Dai, W.; Wang, Z.; Yan, C.; Li, H.; Lin, C.-T.; Jiang, N.; Yu, J. Constructing a “pea-pod-like” alumina-graphene binary architecture for enhancing thermal conductivity of epoxy composite. Chem. Eng. J. 2020, 381, 122690. [Google Scholar] [CrossRef]
- Yang, X.; Fan, S.; Li, Y.; Guo, Y.; Li, Y.; Ruan, K.; Zhang, S.; Zhang, J.; Kong, J.; Gu, J. Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos. Part A 2020, 128, 105670. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Z.; Zou, W.; Li, H.; Zhang, C. MgO nanoparticles-decorated carbon fibers hybrid for improving thermal conductive and electrical insulating properties of Nylon 6 composite. Compos. Sci. Technol. 2017, 148, 1–8. [Google Scholar] [CrossRef]
- Shen, C.; Wang, H.; Zhang, T.; Zeng, Y. Silica coating onto graphene for improving thermal conductivity and electrical insulation of graphene/polydimethylsiloxane nanocomposites. J. Mater. Sci. Technol. 2019, 35, 36–43. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Kong, X.; Gao, D.; Wang, Y.; Hu, T.; Zhang, L. Mussel-inspired modification of boron nitride for natural rubber composites with high thermal conductivity and low dielectric constant. Compos. Sci. Technol. 2019, 177, 18–25. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Ruan, K.; Zhang, H.; Gu, J. Highly thermally conductive carbon nanotubes pillared exfoliated graphite/polyimide composites. NPJ Flex. Electron. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Jiang, F.; Cui, X.; Song, N.; Shi, L.; Ding, P. Synergistic effect of functionalized graphene/boron nitride on the thermal conductivity of polystyrene composites. Compos. Commun. 2020, 20, 100350. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Lee, H.; Kim, H.-T.; Lee, Y.M.; Ryou, M.-H. Polydopamine-treated three-dimensional carbon fiber-coated separator for achieving high-performance lithium metal batteries. J. Power Sources 2019, 430, 130–136. [Google Scholar] [CrossRef]
- Dong, X.; Ding, B.; Guo, H.; Dou, H.; Zhang, X. Superlithiated polydopamine derivative for high-capacity and high-rate anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 38101–38108. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Q.; Xie, D.; Liu, J.; Chen, X.; Nan, J.; Zuo, X. Low-cost and heat-resistant poly(catechol/polyamine)-silica composite membrane for high-performance lithium-ion batteries. ChemElectroChem 2021, 8, 1369–1376. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.; Ruan, K.; Kong, J.; Dong, M.; Zhang, J.; Gu, J.; Guo, Z. Reduced graphene oxide heterostructured silver nanoparticles significantly enhanced thermal conductivities in hot-pressed electrospun polyimide nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 25465–25473. [Google Scholar] [CrossRef]
- Xiong, S.-W.; Zhang, P.; Xia, Y.; Zou, Q.; Jiang, M.; Gai, J.-G. Unique antimicrobial/thermally conductive polymer composites for use in medical electronic devices. J. Appl. Polym. Sci. 2021, 138, 50113. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, D.; Yu, L.; Ni, Y.; Zhang, L. Fabrication of carboxyl nitrile butadiene rubber composites with high dielectric constant and thermal conductivity using Al2O3@PCPA@GO hybrids. Compos. Sci. Technol. 2020, 199, 108344. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, J.; Feng, Y.; Feng, W. Assembly of graphene-aligned polymer composites for thermal conductive applications. Compos. Commun. 2018, 9, 33–41. [Google Scholar] [CrossRef]
- Xiao, C.; Song, Q.; Shen, Q.; Wang, T.; Xie, W. Understanding on interlaminar nano-reinforcement induced mechanical performance improvement of carbon/carbon composites after silicon infiltration. Compos. Part B Eng. 2022, 239, 109946. [Google Scholar] [CrossRef]
- Han, Y.; Ruan, K.; Gu, J. Janus (BNNS/ANF)-(AgNWs/ANF) thermal conductivity composite films with superior electromagnetic interference shielding and joule heating performances. Nano Res. 2022, 15, 4747–4755. [Google Scholar] [CrossRef]
- Cetin, M.S.; Toprakci, H.A.K. Flexible electronics from hybrid nanocomposites and their application as piezoresistive strain sensors. Compos. Part B Eng. 2021, 224, 109199. [Google Scholar] [CrossRef]
- Yu, L.; Yang, D.; Wei, Q.; Zhang, L. Constructing of strawberry-like core-shell structured Al2O3 nanoparticles for improving thermal conductivity of nitrile butadiene rubber composites. Compos. Sci. Technol. 2021, 209, 108786. [Google Scholar] [CrossRef]
- Ji, S.-Y.; Jung, H.-B.; Kim, M.-K.; Lim, J.-H.; Kim, J.-Y.; Ryu, J.; Jeong, D.-Y. Enhanced energy storage performance of polymer/ceramic/metal composites by increase of thermal conductivity and coulomb-blockade effect. ACS Appl. Mater. Interfaces 2021, 13, 27343–27352. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Singh, M.; Das, P.; Pradhan, N.; Al-Enizi, A.M.; Karim, A.; Raghavan, D. Recent developments in the synthesis of chemically modified nanomaterials for use in dielectric and electronics applications. Nanotechnology 2021, 32, 142004. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xie, C.; Luo, S.; Xu, H.; Gou, B.; Zeng, L. Preparation and properties of MWCNTs-BNNSs/epoxy composites with high thermal conductivity and low dielectric loss. Mater. Today Commun. 2020, 24, 100985. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Wang, Q.; Zhang, W.; Song, S.; Li, X.; Li, Y.; Li, B. Enhanced thermal conductivity by constructing 3D-networks in poly(vinylidene fluoride) composites via positively charged hexagonal boron nitride and silica coated carbon nanotubes. Compos. Part A: Appl. Sci. Manuf. 2020, 137, 106038. [Google Scholar] [CrossRef]
- Wang, B.; Yin, X.H.; Peng, D.; Lv, R.H.; Na, B.; Liu, H.S.; Gu, X.B.; Wu, W.; Zhou, J.L.; Zhang, Y. Achieving thermally conductive low loss PVDF-based dielectric composites via surface functionalization and orientation of SiC nanowires. Express Polym. Lett. 2020, 14, 2–11. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Q.; Pang, X.; Gong, B.; Wei, C.; Ren, J. Synergistic enhanced thermal conductivity and dielectric constant of epoxy composites with mesoporous silica coated carbon nanotube and boron nitride nanosheets. Materials 2021, 14, 5251. [Google Scholar] [CrossRef]
- Schönhals, A.; Kremer, F. Analysis of Dielectric Spectra; Springer: Berlin/Heidelberg, Germany, 2003; pp. 59–98. [Google Scholar]
- Shen, Z.; Feng, J. Achieving vertically aligned SiC microwires networks in a uniform cold environment for polymer composites with high through-plane thermal conductivity enhancement. Compos. Sci. Technol. 2019, 170, 135–140. [Google Scholar] [CrossRef]
- Ngo, I.L.; Byon, C.; Lee, B.J. Analytical study on thermal conductivity enhancement of hybrid-filler polymer composites under high thermal contact resistance. Int. J. Heat Mass Transf. 2018, 126, 474–484. [Google Scholar] [CrossRef]
- Ngo, I.L.; Vattikuti, P.S.V.; Byon, C. A modified Hashin-Shtrikman model for predicting the thermal conductivity of polymer composites reinforced with randomly distributed hybrid fillers. Int. J. Heat Mass Tranf. 2017, 114, 727–734. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Shang, Y.; Zhu, X.; Jiang, Z.; Zhou, C.; Han, J.; Zhang, H. Non-covalent modification of boron nitride nanoparticle-reinforced PEEK composite: Thermally conductive, interfacial, and mechanical properties. Polymer 2020, 203, 122763. [Google Scholar] [CrossRef]
- Du, C.; Li, M.; Cao, M.; Song, S.; Feng, S.; Li, X.; Guo, H.; Li, B. Mussel-inspired and magnetic co-functionalization of hexagonal boron nitride in poly(vinylidene fluoride) composites toward enhanced thermal and mechanical performance for heat exchangers. ACS Appl. Mater. Interfaces 2018, 10, 34674–34682. [Google Scholar] [CrossRef]









| Samples | Elemental Analysis (wt%) | |||
|---|---|---|---|---|
| B | N | C | O | |
| hBN | 50.54 | 39.17 | 7.79 | 2.50 |
| mhBN | 46.07 | 38.07 | 13.03 | 2.83 |
| mhBN@GO | 40.67 | 34.48 | 19.31 | 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Yang, D. Achieving High Thermal Conductivity and Satisfactory Insulating Properties of Elastomer Composites by Self-Assembling BN@GO Hybrids. Polymers 2023, 15, 523. https://doi.org/10.3390/polym15030523
Xie X, Yang D. Achieving High Thermal Conductivity and Satisfactory Insulating Properties of Elastomer Composites by Self-Assembling BN@GO Hybrids. Polymers. 2023; 15(3):523. https://doi.org/10.3390/polym15030523
Chicago/Turabian StyleXie, Xing, and Dan Yang. 2023. "Achieving High Thermal Conductivity and Satisfactory Insulating Properties of Elastomer Composites by Self-Assembling BN@GO Hybrids" Polymers 15, no. 3: 523. https://doi.org/10.3390/polym15030523