Facile Preparation of Cobalt Nanoparticles Encapsulated Nitrogen-Doped Carbon Sponge for Efficient Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
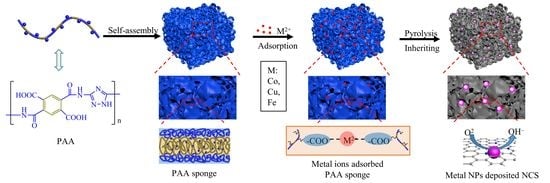
2.2. Synthesis of Poly(amic acid) (PAA)
2.3. Self-Assembly of PAA into Sponge
2.4. Preparation of Co, Cu, and Co/Cu Nanoparticles-Loaded Nitrogen-Doped Carbon Sponge (M@NCS)
2.5. Electrochemical Measurements
2.6. Characterizations
3. Results and Discussion
3.1. Synthesis and Self-Assembly of PAA
3.2. Preparation and Characterization of M@NCS
3.3. Electrocatalytic Performance toward ORR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium—Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D.Y. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Cleghorn, S.J.C.; Ren, X.; Springer, T.E.; Wilson, M.S.; Zawodzinski, C.; Zawodzinski, T.A.; Gottesfeld, S. PEM fuel cells for transportation and stationary power generation applications. Int. J. Hydrogen Energy 1997, 22, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chutia, A.; Brett, D.J.L.; Shearing, P.R.; He, G.; Chai, G.; Parkin, I.P. Palladium alloys used as electrocatalysts for the oxygen reduction reaction. Energy Environ. Sci. 2021, 14, 2639–2669. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.-C.; Wu, Z.-P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.-C.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Norskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.B.; Zou, R.Q.; Guo, S.J. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef]
- Dou, S.; Wang, X.; Wang, S.Y. Rational Design of Transition Metal-Based Materials for Highly Efficient Electrocatalysis. Small Methods 2019, 3, 1800211. [Google Scholar] [CrossRef]
- Chang, H.; Joo, S.H.; Pak, C. Synthesis and characterization of mesoporous carbon for fuel cell applications. J. Mater. Chem. 2007, 17, 3078–3088. [Google Scholar] [CrossRef]
- Liu, H.S.; Song, C.J.; Zhang, L.; Zhang, J.J.; Wang, H.J.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Zhao, S.; Ban, L.; Zhang, J.; Yi, W.; Sun, W.; Zhu, Z. Cobalt and nitrogen co-doping of porous carbon nanosphere as highly effective catalysts for oxygen reduction reaction and Zn-air battery. Chem. Eng. J. 2021, 409, 128171. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Leung, M.K.H. Valence Engineering of Polyvalent Cobalt Encapsulated in a Carbon Nanofiber as an Efficient Trifunctional Electrocatalyst for the Zn-Air Battery and Overall Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 4399–4408. [Google Scholar]
- Liu, S.; Yang, H.; Yao, L.; Peng, H.; Huang, P.; Lin, X.; Liu, L.; Zhang, H.; Cai, P.; Wen, X.; et al. Design of Fe and Cu bimetallic integration on N and F co-doped porous carbon material for oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 7751–7760. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.Y.; Hao, Y.; Shao, Z.P.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, H.E.; Han, D.; Lee, J.H. Low-temperature fabrication of crystalline MnCoO spinel film on porous carbon paper for efficient oxygen evolution reaction. Chem. Commun. 2021, 57, 3595–3598. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Y.; Fan, J.; Wang, L.; Lin, Y.; Xu, D.; Dai, Z.; Han, M. Engineering bimetal Cu, Co sites on 3D N-doped porous carbon nanosheets for enhanced oxygen reduction electrocatalysis. Chem. Commun. 2020, 56, 10010–10013. [Google Scholar]
- Chen, Z.; Liu, R.; Liu, S.; Huang, J.; Chen, L.; Nadimicherla, R.; Wu, D.; Fu, R. FeS/FeNC decorated N,S-co-doped porous carbon for enhanced ORR activity in alkaline media. Chem. Commun. 2020, 56, 12921–12924. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Dodelet, J.P.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, 1807615. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wang, B.; Tao, L.; Cunning, B.V.; Zhang, Z.P.; Wang, S.Y.; Ruoff, R.S.; Qu, L.T. Efficient Metal-Free Electrocatalysts from N-Doped Carbon Nanomaterials: Mono-Doping and Co-Doping. Adv. Mater. 2019, 31, 1805121. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.Q.; Hao, J.Y.; Liu, Y.S.; Li, W.Z.; Li, J. N and P co-functionalized three-dimensional porous carbon networks as efficient metal-free electrocatalysts for oxygen reduction reaction. Carbon 2017, 122, 64–73. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L.M. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Gong, K.P.; Du, F.; Xia, Z.H.; Durstock, M.; Dai, L.M. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Ying, Y.R.; Sun, D.R.; Zhang, Z.Y.; Fei, L.F.; Wen, Z.H.; Qiao, J.L.; Huang, H.T. Highly Efficient Porous Carbon Electrocatalyst with Controllable N-Species Content for Selective CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 3244–3251. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, J.; Tang, Z.; Bi, D.; Zhao, J.; Liang, Y. Optimal Configuration of N-Doped Carbon Defects in 2D Turbostratic Carbon Nanomesh for Advanced Oxygen Reduction Electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 11999–12006. [Google Scholar] [CrossRef]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.M.; Chen, Y. Observation of Active Sites for Oxygen Reduction Reaction on Nitrogen-Doped Multilayer Graphene. ACS Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Huang, Y.L.; Zhang, Y.Y.; Peng, W.; Xia, S.H.; Yu, J.Y.; Ding, B. Facile Synthesis of Bimetallic Fluoride Heterojunctions on Defect-Enriched Porous Carbon Nanofibers for Efficient ORR Catalysts. Nano Lett. 2021, 21, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.D.; Ji, D.X.; Zhang, S.; He, X.W.; Ramakrishna, S.; Zhang, Q.Y. A Humidity-Induced Nontemplating Route toward Hierarchical Porous Carbon Fiber Hybrid for Efficient Bifunctional Oxygen Catalysis. Small 2020, 16, 2001743. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Huang, N.B.; Ge, X.W.; Xuan, J.P.; Wang, P. Preparation of N-doped nano-hollow capsule carbon nanocage as ORR catalyst in alkaline solution by PVP modified F127. Int. J. Hydrogen Energy 2020, 45, 8667–8675. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Jin, K.; Lai, X.Y.; Du, J.Z. Highly porous nitrogen-doped carbon superstructures derived from the intramolecular cyclization-induced crystallization-driven self-assembly of poly(amic acid). Nanoscale Adv. 2022, 4, 1422–1430. [Google Scholar] [CrossRef]
- Liu, S.N.; Zhao, T.Q.; Tan, X.H.; Guo, L.M.; Wu, J.X.; Kang, X.H.; Wang, H.F.; Sun, L.F.; Chu, W.G. 3D pomegranate-like structures of porous carbon microspheres self-assembled by hollow thin-walled highly-graphitized nanoballs as sulfur immobilizers for Li-S batteries. Nano Energy 2019, 63, 103894. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, Y.; Yang, B.; Wang, Y.; Wu, Y.; Du, J. Template-free fabrication of nitrogen-doped hollow carbon spheres for high-performance supercapacitors based on a scalable homopolymer vesicle. J. Mater. Chem. A 2016, 4, 12088–12097. [Google Scholar] [CrossRef]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Sun, H.; Du, J. Intramolecular Cyclization-Induced Crystallization-Driven Self-Assembly of an Amorphous Poly(amic acid). Macromolecules 2020, 53, 11033–11039. [Google Scholar] [CrossRef]
- Sun, H.; Chen, S.; Li, X.; Leng, Y.; Zhou, X.; Du, J. Lateral growth of cylinders. Nat. Commun. 2022, 13, 2170. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, X.; Leng, Y.; Li, X.; Du, J. Transformation of Amorphous Nanobowls to Crystalline Ellipsoids Induced by Trans-Cis Isomerization of Azobenzene. Macromol. Rapid Commun. 2022, 43, 2200131. [Google Scholar] [CrossRef]
- Sun, H.; Leng, Y.; Zhou, X.; Li, X.; Wang, T. Regulation of the nanostructures self-assembled from an amphiphilic azobenzene homopolymer: Influence of initial concentration and solvent solubility parameter. Soft Matter, 2023; ahead of print. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Xie, Z.; Qiu, Y.; Zhuo, L.; Qin, Y.; Ren, J.; Zhang, S.; Hu, G.; Luo, J.; et al. Metal-Free Bifunctional Ordered Mesoporous Carbon for Reversible Zn-CO2 Batteries. Small Methods 2021, 5, 2001039. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.; Wei, C.; Sun, Z.; Zeng, K.; Shen, L.; Sun, J.; Rummeli, M.H.; Yang, R. Nitrogen-doped hollow carbon polyhedron derived from salt-encapsulated ZIF-8 for efficient oxygen reduction reaction. Carbon 2021, 171, 320–328. [Google Scholar] [CrossRef]
- Zhong, X.; Ye, S.; Tang, J.; Zhu, Y.; Wu, D.; Gu, M.; Pan, H.; Xu, B. Engineering Pt and Fe dual-metal single atoms anchored on nitrogen-doped carbon with high activity and durability towards oxygen reduction reaction for zinc-air battery. Appl. Catal. B 2021, 286, 119891. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Luo, Z.; Veder, J.-P.; Zhong, Y.; Jiang, S.P.; Shao, Z. Defects-rich porous carbon microspheres as green electrocatalysts for efficient and stable oxygen-reduction reaction over a wide range of pH values. Chem. Eng. J. 2021, 406, 126883. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; Yu, M.; Wang, W. Trifunctional Co nanoparticle confined in defect-rich nitrogen-doped graphene for rechargeable Zn-air battery with a long lifetime. Appl. Catal. B 2021, 281, 119514. [Google Scholar] [CrossRef]
- Sun, H.; Jin, K.; Li, X.; Wang, T.; Lai, X. Nitrogen-Doped Carbon Sponge Derived from the Self-Assembly of a Poly(amic acid) for High Performance Oxygen Reduction Reaction. New J. Chem. 2023; accepted. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Y.; Jin, K.; Wang, T.; Sun, H. Facile Preparation of Cobalt Nanoparticles Encapsulated Nitrogen-Doped Carbon Sponge for Efficient Oxygen Reduction Reaction. Polymers 2023, 15, 521. https://doi.org/10.3390/polym15030521
Leng Y, Jin K, Wang T, Sun H. Facile Preparation of Cobalt Nanoparticles Encapsulated Nitrogen-Doped Carbon Sponge for Efficient Oxygen Reduction Reaction. Polymers. 2023; 15(3):521. https://doi.org/10.3390/polym15030521
Chicago/Turabian StyleLeng, Ying, Kai Jin, Tian Wang, and Hui Sun. 2023. "Facile Preparation of Cobalt Nanoparticles Encapsulated Nitrogen-Doped Carbon Sponge for Efficient Oxygen Reduction Reaction" Polymers 15, no. 3: 521. https://doi.org/10.3390/polym15030521