Synthesis and Characterization of Benzene- and Triazine-Based Azo-Bridged Porous Organic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Synthetic Procedures
2.2.1. Synthesis of AZO-B-P1
2.2.2. Synthesis of AZO-T-P2
2.2.3. Synthesis of AZO-T-P3
2.2.4. Synthesis of AZO-B-P4
2.3. Computational Methods
3. Results and Discussion
3.1. Synthesis of Azo-Bridged Polymers
3.2. Characterization of Azo-Bridged Polymers
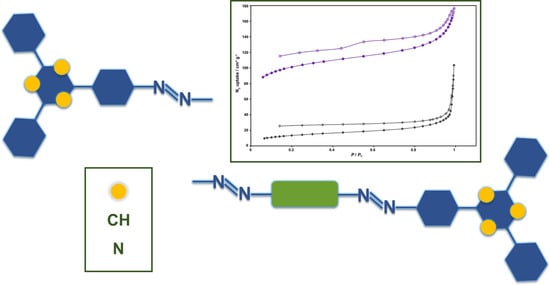
3.3. Porosity Properties of Azo-Bridged Polymers
3.4. Computational Studies of Azo-Bridged Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous Organic Materials: Strategic Design and Structure-Function Correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347. [Google Scholar] [CrossRef]
- Sarkar, C.; Shit, S.C.; Das, N.; Mondal, J. Presenting porous-organic-polymers as next-generation invigorating materials for nanoreactors. Chem. Commun. 2021, 57, 8550–8567. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous Organic Polymers for CO2 Storage and Conversion Reactions. ChemCatChem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Huang, J.; Liu, Y.-N. Synthesis of Triazine-Based Porous Organic Polymers Derived N-Enriched Porous Carbons for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 2856–2865. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Krishna, R.; Yao, K.; Han, Y.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Space, B.; et al. Introduction of π-Complexation into Porous Aromatic Framework for Highly Selective Adsorption of Ethylene over Ethane. J. Am. Chem. Soc. 2014, 136, 8654–8660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Yang, D.-H.; Tao, Y.; Ding, X.; Han, B.-H. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.F.; Lai, W.-Y.; Huang, W. Porous Organic Polymers as Promising Electrode Materials for Energy Storage Devices. Adv.Mater. Technol. 2020, 5, 2000154. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Wang, C.; Luo, X.; Kim, J.; Wang, Z.; Yamauchi, Y. Porous Organic Frameworks: Advanced Materials in Analytical Chemistry. Adv. Sci. 2018, 5, 1801116. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, P.; Zhang, X.; Wu, D. Emerging porous organic polymers for biomedical applications. Chem. Soc. Rev. 2022, 51, 1377–1414. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, M.; Alley, R.B. The West Antarctic ice sheet and long term climate policy. Clim. Chang. 2004, 64, 1–10. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Ann. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Long, H.; Zhang, W. Imine-Linked Porous Polymer Frameworks with High Small Gas (H2, CO2, CH4, C2H2) Uptake and CO2/N2 Selectivity. Chem. Mater. 2013, 25, 1630–1635. [Google Scholar] [CrossRef]
- Gu, G.; Liu, D.; Huang, W.; Liu, J.; Yang, R. Synthesis of covalent triazine-based frameworks with high CO2 adsorption and selectivity. Polym. Chem. 2015, 6, 7410–7417. [Google Scholar] [CrossRef]
- Zhu, X.; Mahurin, S.M.; An, S.-H.; Do-Thanh, C.-L.; Tian, C.; Li, Y.; Gill, L.W.; Hagaman, E.W.; Bian, Z.; Zhou, J.-H.; et al. Efficient CO2 capture by a task-specific porous organic polymer bifunctionalized with carbazole and triazine groups. Chem. Commun. 2014, 50, 7933–7936. [Google Scholar] [CrossRef]
- Nandi, S.; Werner-Zwanzigerb, U.; Vaidhyanathan, R. A triazine-resorcinol based porous polymer with polar pores and exceptional surface hydrophobicity showing CO2 uptake under humid conditions. J. Mater. Chem. A 2015, 3, 21116–21122. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Guo, J.; Huang, Q.; He, J.; Fu, Y.; Kuang, G.; Pan, C.; Yu, G. 1,3,5-Triazine-Based Microporous Polymers with Tunable Porosities for CO2 Capture and Fluorescent Sensing. Macromolecules 2017, 50, 8512–8520. [Google Scholar] [CrossRef]
- Kumar, S.; Hassan, A.; Das, N.; Koh, J. Triazine based nanoarchitectonics of porous organic polymers for CO2 storage. Mater. Lett. 2022, 313, 131757. [Google Scholar] [CrossRef]
- Rabbani, M.G.; El-Kaderi, H.M. Synthesis and Characterization of Porous Benzimidazole-Linked Polymers and Their Performance in Small Gas Storage and Selective Uptake. Chem. Mater. 2012, 24, 1511–1517. [Google Scholar] [CrossRef]
- Dawson, R.; Stöckel, E.; Holst, J.R.; Adams, J.D.; Cooper, I.A. Microporous organic polymers for carbon dioxide capture. Energy Environ. Sci. 2011, 4, 4239–4245. [Google Scholar] [CrossRef]
- Patel, H.A.; Je, S.H.; Park, J.; Jung, Y.; Coskun, A.; Yavuz, C.T. Directing the Structural Features of N2-Phobic Nanoporous Covalent Organic Polymers for CO2 Capture and Separation. Chem. Eur. J. 2014, 20, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Puthiaraj, P.; Lee, Y.-R.; Zhang, S.; Ahn, W.-S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. 2016, 4, 16288–16311. [Google Scholar] [CrossRef]
- Patel, H.A.; Je, S.H.; Park, J.; Chen, D.P.; Jung, Y.; Yavuz, C.T.; Coskun, A. Unprecedented high-temperature CO2 selectivity in N2-phobic nanoporous covalent organic polymers. Nat. Commun. 2013, 4, 1357. [Google Scholar] [CrossRef] [Green Version]
- Arab, P.; Rabbani, M.G.; Sekizkardes, A.K.; İslamoğlu, T.; El-Kaderi, H.M. Copper(I)-Catalyzed Synthesis of Nanoporous Azo-Linked Polymers: Impact of Textural Properties on Gas Storage and Selective Carbon Dioxide Capture. Chem. Mater. 2014, 26, 1385–1392. [Google Scholar] [CrossRef]
- Arab, P.; Parrish, E.; İslamoğlu, T.; El-Kaderi, H.M. Synthesis and evaluation of porous azo-linked polymers for carbon dioxide capture and separation. J. Mater. Chem. A 2015, 3, 20586–20594. [Google Scholar] [CrossRef]
- Bera, R.; Ansari, M.; Alam, A.; Das, N. Triptycene, Phenolic-OH, and Azo-Functionalized Porous Organic Polymers: Efficient and Selective CO2 Capture. ACS Appl. Polym. Mater. 2019, 1, 959–968. [Google Scholar] [CrossRef]
- Abdelnaby, M.M.; Saleh, T.A.; Zeama, M.; Abdalla, M.A.; Ahmed, H.M.; Habib, M.A. Azo-Linked Porous Organic Polymers for Selective Carbon Dioxide Capture and Metal Ion Removal. ACS Omega 2022, 7, 14535–14543. [Google Scholar] [CrossRef]
- Tao, L.; Niu, F.; Zhang, D.; Liu, J.; Wang, T.; Wang, Q. Azo-bridged covalent porphyrinic polymers (Azo-CPPs): Synthesis and CO2 capture properties. RSC Adv. 2015, 5, 96871–96878. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J. Facile synthesis of azo-linked porous organic frameworks via reductive homocoupling for selective CO2 capture. J. Mater. Chem. A 2014, 2, 13831–13834. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Luo, X.-S.; Liu, X.; Qiao, Y.; Wang, P.; Mecerreyes, D.; Bogliotti, N.; Chen, S.-L.; Huang, M.-H. Azo-linked porous organic polymers: Robust and time-efficient synthesis via NaBH4-mediated reductive homocoupling on polynitro monomers and adsorption capacity towards aniline in water. J. Mater. Chem. A 2018, 6, 5608–5612. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z. Microporous Polyimides with Uniform Pores for Adsorption and Separation of CO2 Gas and Organic Vapors. Macromolecules 2013, 46, 3058–3066. [Google Scholar] [CrossRef]
- Halder, A.; Kandambeth, S.; Biswal, B.P.; Kaur, G.; Roy, N.C.; Addicoat, M.; Salunke, J.K.; Banerjee, S.; Vanka, K.; Heine, T.; et al. Decoding the Morphological Diversity in Two Dimensional Crystalline Porous Polymers by Core Planarity Modulation. Angew. Chem. Int. Ed. 2016, 55, 7806–7810. [Google Scholar] [CrossRef]
- Šutalo, P.; Pisačić, M.; Biljan, I.; Kodrin, I. Benzene and Triazine-Based Porous Organic Polymers with Azo, Azoxy and Azodioxy Linkages: A Computational Study. CrystEngComm 2022, 24, 4748–4763. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Spek, A.L. CheckCIF Validation ALERTS: What They Mean and How to Respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Oliveira, D.V.; Laun, J.; Peintinger, M.F.; Bredow, T. BSSE-Correction Scheme for Consistent Gaussian Basis Sets of Double- and Triple-Zeta Valence with Polarization Quality for Solid-State Calculations. J. Comput. Chem. 2019, 40, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical Condensed Matter Simulations with CRYSTAL. WIREs Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Björkman, T. CIF2Cell: Generating Geometries for Electronic Structure Programs. Comput. Phys. Commun. 2011, 182, 1183–1186. [Google Scholar] [CrossRef]
- Campañá, C.; Mussard, B.; Woo, T.K. Electrostatic Potential Derived Atomic Charges for Periodic Systems Using a Modified Error Functional. J. Chem. Theory Comput. 2009, 5, 2866–2878. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular Simulation Software for Adsorption and Diffusion in Flexible Nanoporous Materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.G.; Siepmann, J.I. Transferable Potentials for Phase Equilibria. 1. United-Atom Description of n-Alkanes. J. Phys. Chem. B 1998, 102, 2569–2577. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A Generic Force Field for Molecular Simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Wisser, F.M.; Eckhardt, K.; Wisser, D.; Böhlmann, W.; Grothe, J.; Brunner, E.; Kaskel, S. Tailoring Pore Structure and Properties of Functionalized Porous Polymers by Cyclotrimerization. Macromolecules 2014, 47, 4210–4216. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Kenneth, S.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Das, S.K.; Bhanja, P.; Kundu, S.K.; Mondal, S.; Bhaumik, A. Role of Surface Phenolic-OH Groups in N-Rich Porous Organic Polymers for Enhancing the CO2 Uptake and CO2/N2 Selectivity: Experimental and Computational Studies. ACS Appl. Mater. Interfaces 2018, 10, 23813–23824. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-W.; Su, F.; Trewin, A.; Wood, C.D.; Niu, H.; Jones, J.T.A.; Khimyak, Y.Z.; Cooper, A.I. Synthetic Control of the Pore Dimension and Surface Area in Conjugated Microporous Polymer and Copolymer Networks. J. Am. Chem. Soc. 2008, 130, 7710–7720. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, W.; Feng, S.; Liu, H. Constructing hybrid porous polymers from cubic octavinylsilsequioxane and planar halogenated benzene. Polym. Chem. 2014, 5, 3634–3642. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.; Li, J.; Ma, Q. Porous Organic Polymers Derived from Ferrocene and Tetrahedral Silicon-Centered Monomers for Carbon Dioxide Sorption. Polymers 2022, 14, 370. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Kuo, C.H.; Alshehri, A.; Young, C.; Yamauchi, Y.; Kim, J.; Kuo, S.W. Strategic Design of Triphenylamine- and Triphenyltriazine-Based Two-Dimensional Covalent Organic Frameworks for CO2 Uptake and Energy Storage. J. Mater. Chem. A 2018, 6, 19532–19541. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Z.; Wee, V.; Usadi, A.K.; Calabro, D.C.; Baugh, L.S.; Wang, S.; Wang, Y.; Zhao, D. Interlayer Shifting in Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12995–13002. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, X.; Liu, Y.; Liu, Y.; Cui, Y. Control Interlayer Stacking and Chemical Stability of Two-Dimensional Covalent Organic Frameworks via Steric Tuning. J. Am. Chem. Soc. 2018, 140, 16124–16133. [Google Scholar] [CrossRef]






| Sample | SBET (m2 g−1) |
|---|---|
| AZO-B-P1 | 2.40 |
| AZO-T-P2 | 351 |
| AZO-T-P3 | 50.8 |
| AZO-B-P4 | 0.74 |
| AZO-T-P5 | 0.44 |
| AZO-B-P6 | 0.43 |
| AZO-B-P7 | 0.42 |
| AZO-B-P8 | 0.83 |
| AZO-B-P9 | 0.60 |
| AZO-B-P10 | 0.63 |
| AZO-B-P11 | 0.41 |
| AZO-T-P12 | 0.05 |
| AZO-T-P13 | 0.20 |
| AZO-T-P14 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panić, B.; Frey, T.; Borovina, M.; Konopka, K.; Sambolec, M.; Kodrin, I.; Biljan, I. Synthesis and Characterization of Benzene- and Triazine-Based Azo-Bridged Porous Organic Polymers. Polymers 2023, 15, 229. https://doi.org/10.3390/polym15010229
Panić B, Frey T, Borovina M, Konopka K, Sambolec M, Kodrin I, Biljan I. Synthesis and Characterization of Benzene- and Triazine-Based Azo-Bridged Porous Organic Polymers. Polymers. 2023; 15(1):229. https://doi.org/10.3390/polym15010229
Chicago/Turabian StylePanić, Barbara, Tea Frey, Mladen Borovina, Kristijan Konopka, Miro Sambolec, Ivan Kodrin, and Ivana Biljan. 2023. "Synthesis and Characterization of Benzene- and Triazine-Based Azo-Bridged Porous Organic Polymers" Polymers 15, no. 1: 229. https://doi.org/10.3390/polym15010229