Macro-Size Regenerated Cellulose Fibre Embedded with Graphene Oxide with Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
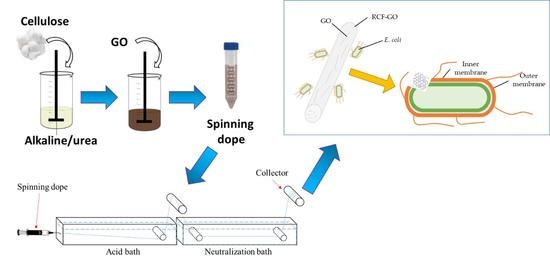
2.2. Preparation of Cellulose/GO Fibre
2.3. Raman Spectroscopy
2.4. Transmission Electron Microscopy (TEM)
2.5. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.6. X-Ray Diffractometry (XRD) Analysis
2.7. Field-Emission Scanning Electron Microscopy (FESEM)
2.8. Dino-Lite Microscopy
2.9. Thermogravimetric Analysis
2.10. Mechanical Testing of the RCFs
2.11. Antibacterial Analysis
3. Results
3.1. Characterisation of GO
3.2. FTIR Analysis
3.3. Morphology of RCFs
3.4. XRD Analysis
3.5. Thermal Analysis
3.6. Mechanical Properties of RCF
3.7. Antibacterial Activity of RCFs with and without GO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, K.; Netravali, A.N. Fabrication and Characterization of Biodegradable Composites Based on Microfibrillated Cellulose and Polyvinyl Alcohol. Compos. Sci. Technol. 2012, 72, 1588–1594. [Google Scholar] [CrossRef]
- Jafri, N.F.; Salleh, K.M.; Zakaria, S.; Hassan, N.J. Penghasilan Filem Selulosa Terjana Semula: Suatu Ulasan (Production of Regenerated Cellulose Film: A Review). Sains Malaysiana 2022, 51, 1525–1543. [Google Scholar] [CrossRef]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Ayob, M.K.; Huang, N.M.; Neoh, H.M.; He, M.; Zhang, L.; Jamal, R. A Graphene Oxide Facilitated a Highly Porous and Effective Antibacterial Regenerated Cellulose Membrane Containing Stabilized Silver Nanoparticles. Cellulose 2014, 21, 4261–4270. [Google Scholar] [CrossRef]
- Mazlan, N.S.N.; Zakaria, S.; Gan, S.; Hua, C.C.; Baharin, K.W. Comparison of Regenerated Cellulose Membrane Coagulated in Sulphate Based Coagulant. Cerne 2019, 25, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Armir, N.A.Z.; Salleh, K.M.; Zulkifli, A.; Zakaria, S. pH-Responsive Ampholytic Regenerated Cellulose Hydrogel Integrated with Carrageenan and Chitosan. Ind. Crops. Prod. 2022, 178. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S. Superabsorbent Hydrogel from Oil Palm Empty Fruit Bunch Cellulose and Sodium Carboxymethylcellulose. Int. J. Biol. Macromol. 2019, 131, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, P.; Zhang, Y.; Zhang, X.; Jiang, Y. From Waste Cotton Linter: A Renewable Environment-Friendly Biomass Based Carbon Fibers Preparation. ACS Sustain. Chem. Eng. 2016, 4, 5585–5593. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; Michud, A.; Sixta, H. Dry Jet-Wet Spinning of Strong Cellulose Filaments from Ionic Liquid Solution. Cellulose 2017, 24, 3109–3110. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudian, S.; Wahit, M.U.; Ismail, A.F.; Yussuf, A.A. Preparation of Regenerated Cellulose/Montmorillonite Nanocomposite Films via Ionic Liquids. Carbohydr. Polym. 2012, 88, 1251–1257. [Google Scholar] [CrossRef]
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film Properties from Crosslinking of Cellulosic Derivatives with a Polyfunctional Carboxylic Acid. Carbohydr. Polym. 2002, 51, 265–271. [Google Scholar] [CrossRef]
- Tsai, T.T.; Huang, T.H.; Chang, C.J.; Yi-Ju Ho, N.; Tseng, Y.T.; Chen, C.F. Antibacterial Cellulose Paper Made with Silver-Coated Gold Nanoparticles. Sci. Rep. 2017, 7, 3155. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Chang, C.; Wang, Y. Recent Developments and Prospective Food-Related Applications of Cellulose Nanocrystals: A Review. Cellulose 2020, 27, 2991–3011. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Lustri, W.R.; Gomes, H.; Barud, D.O.; Barud, S.; Peres, M.F.S.; Gutierrez, J.; Tercjak, A. Microbial Cellulose—Biosynthesis Mechanisms and Medical Applications. In Cellulose-Fundamental Aspects and Current Trends; Poletto, M., Ed.; IntechOpen: Londan, UK, 2015; pp. 13–157. [Google Scholar]
- Orlando, I.; Roy, I. Cellulose-Based Hydrogels for Wound Healing; Springer: Cham, Switzerland, 2019; pp. 1131–1148. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, Z.; Luo, X.; Zhang, H.; Liu, Y.; Cai, N.; Xue, Y.; Yu, F. Citrate-Modified Maghemite Enhanced Binding of Chitosan Coating on Cellulose Porous Membranes for Potential Application as Wound Dressing. Carbohydr. Polym. 2017, 166, 320–328. [Google Scholar] [CrossRef]
- Abrigo, M.; Mcarthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; Maulucci, G.; De Spirito, M. The Future Development of Bacteria Fighting Medical Devices: The Role of Graphene Oxide. Expert Rev. Med. Devices 2016, 13, 1013–1019. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial Activity of Graphene Oxide Nanosheet against Multidrug Resistant Superbugs Isolated from Infected Patients: Graphene Oxide Antibacterial Activity. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef]
- Wu, G.; Xu, X.; He, X.; Yan, Y. Preparation and Characterization of Graphene Oxide-Modified Sapium Sebiferum Oil-Based Polyurethane Composites with Improved Thermal and Mechanical Properties. Polymers 2018, 10, 133. [Google Scholar] [CrossRef]
- Fu, J.; Wei, C.; Wang, W.; Wei, J.L.; Lv, J. Studies of Structure and Properties of Graphene Oxide Prepared by Ball Milling. Mater. Res. Innov. 2015, 19, S1-277–S1-280. [Google Scholar] [CrossRef]
- Cacaci, M.; Martini, C.; Guarino, C.; Torelli, R.; Bugli, F.; Sanguinetti, M. Graphene Oxide Coatings as Tools to Prevent Microbial Biofilm Formation on Medical Device. Adv. Exp. Med. Biol. 2020, 1282, 21–35. [Google Scholar] [CrossRef]
- Cobos, M.; De-la-pinta, I.; Quind, G.; Jes, M. Graphene Oxide—Silver Nanoparticle Nanohybrids: Synthesis, Characterisation, and Antimicrobial Properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Song, L.; Wang, Z.; Wang, Y.; Wan, L.; Yao, J. International Journal of Biological Macromolecules Highly Transparent Graphene Oxide / Cellulose Composite Fi Lm Bearing Ultraviolet Shielding Property. Int. J. Biol. Macromol. 2020, 145, 663–667. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, P. Surface Coating Modified Polyglycolide (PGA) Braided Threads as Potential Thread-Embedding Materials. Fibers Polym. 2020, 21, 2401–2406. [Google Scholar] [CrossRef]
- Khairunnisa-Atiqah, M.K.; Salleh, K.M.; Ainul Hafiza, A.H.; Mazlan, N.S.N.; Zakaria, S. Impact of Drying Regimes and Different Coating Layers on Carboxymethyl Cellulose Cross-Linked with Citric Acid on Cotton Thread Fibers for Wound Dressing Modification. Polymers 2022, 14, 1217. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, X.; Du, D.; He, C. Fabrication of Multilayer Films from Regenerated Cellulose and Graphene Oxide through Layer-by-Layer Assembly. Prog. Nat. Sci. Mater. Int. 2012, 22, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Chen, Y.; Zeng, Z.; Zhu, J.; Wei, Y.; Li, F.; Liu, L. Composites: Part A Effect of ZnO Nanoparticles Doped Graphene on Static and Dynamic Mechanical Properties of Natural Rubber Composites. Compos. Part A 2015, 70, 35–44. [Google Scholar] [CrossRef]
- Lota, G.; Krawczyk, P.; Lota, K.; Sierczyńska, A.; Kolanowski, Ł.; Baraniak, M.; Buchwald, T. The Application of Activated Carbon Modified by Ozone Treatment for Energy Storage. J. Solid State Electrochem. 2016, 20, 2857–2864. [Google Scholar] [CrossRef] [Green Version]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly Exposed {001} Facets of Titanium Dioxide Modified with Reduced Graphene Oxide for Dopamine Sensing. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR Study on Cellulose with the Varied Moisture Contents: Insight into the Supramolecular Structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hojati Marvast, E.; Ashori, A.; Hamzeh, Y.; Karimi, A.N. Preparation of Cellulose/Polyvinyl Alcohol Biocomposite Films Using 1-n-Butyl-3-Methylimidazolium Chloride. Int. J. Biol. Macromol. 2013, 62, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Zakaria, S.; Chia, C.H.; Kaco, H. Effect of Graphene Oxide on Thermal Stability of Aerogel Bio-Nanocomposite from Cellulose-Based Waste Biomass. Cellulose 2018, 25, 5099–5112. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Bai, H.; Dong, W.; Liu, X. All Cellulose Composites Based on Cellulose Diacetate and Nanofibrillated Cellulose Prepared by Alkali Treatment. Carbohydr. Polym. 2018, 179, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, Y.; Shi, C.; Ye, Y.; Wang, P.; Zeng, X.; Wu, T. Preparation and Characterisation of Cellulose Regenerated from Phosphoric Acid. J. Agric. Food Chem. 2013, 61, 12405–12414. [Google Scholar] [CrossRef]
- Xu, L.; Teng, J.; Li, L.; Huang, H.D.; Xu, J.Z.; Li, Y.; Ren, P.G.; Zhong, G.J.; Li, Z.M. Hydrophobic Graphene Oxide as a Promising Barrier of Water Vapor for Regenerated Cellulose Nanocomposite Films. ACS Omega 2019, 4, 509–517. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Wang, H.; Liu, C.; Pan, X. Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers 2018, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.; Zakaria, S.; Nabihah, S.; Jaafar, S. Enhanced Mechanical Properties of Hydrothermal Carbamated Cellulose Nanocomposite Film Reinforced with Graphene Oxide. Carbohydr. Polym. 2017, 172, 284–293. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Zhou, J. Structure and Properties of Regenerated Cellulose Films Prepared from Cotton Linters in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2001, 40, 5923–5928. [Google Scholar] [CrossRef]
- Wang, B.; Lou, W.; Wang, X.; Hao, J. Relationship between Dispersion State and Reinforcement Effect of Graphene Oxide in Microcrystalline Cellulose-Graphene Oxide Composite Films. J. Mater. Chem. 2012, 22, 12859–12866. [Google Scholar] [CrossRef]
- Su, Z.; Sun, D.D.; Zhang, L.; He, M.; Jiang, Y.; Millar, B.; Douglas, P.; Mariotti, D.; Maguire, P.; Sun, D.D. Chitosan/Silver Nanoparticle/Graphene Oxide Nanocomposites with Multi-Drug Release, Antimicrobial, and Photothermal Conversion Functions. Materials 2021, 14, 2351. [Google Scholar] [CrossRef]
- Gabryś, T.M.; Fryczkowska, B.; Machnicka, A.; Graczyk, T. Nanocomposite Cellulose Fibres Doped with Graphene Oxide and Their Biocidal Properties. Polymers 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Gottrup, F.; Cullen, B.M.; Karlsmark, T.; Bischoff-Mikkelsen, M.; Nisbet, L.; Gibson, M.C. Randomized Controlled Trial on Collagen/Oxidized Regenerated Cellulose/Silver Treatment. Wound Repair Regen. 2013, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]











| Sample | Crystallinity Index (%) |
|---|---|
| Cotton linter | 58.89 |
| RCF−0 | 56.94 |
| RCF−GO1 | 57.44 |
| RCF−GO2 | 57.74 |
| RCF−GO3 | 57.58 |
| Sample | To (°C) | Tmax (°C) | Tf (°C) | Residue (%) |
|---|---|---|---|---|
| RCF-0 | 275 | 322 | 345 | 17.98 |
| RCF−GO1 | 287 | 328 | 345 | 27.22 |
| RCF−GO2 | 305 | 328 | 345 | 22.21 |
| RCF−GO3 | 281 | 322 | 345 | 28.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazlan, N.S.N.; Salleh, K.M.; Khairunnisa-Atiqah, M.K.; Ainul Hafiza, A.H.; Mostapha, M.; Ellis, A.V.; Zakaria, S. Macro-Size Regenerated Cellulose Fibre Embedded with Graphene Oxide with Antibacterial Properties. Polymers 2023, 15, 230. https://doi.org/10.3390/polym15010230
Mazlan NSN, Salleh KM, Khairunnisa-Atiqah MK, Ainul Hafiza AH, Mostapha M, Ellis AV, Zakaria S. Macro-Size Regenerated Cellulose Fibre Embedded with Graphene Oxide with Antibacterial Properties. Polymers. 2023; 15(1):230. https://doi.org/10.3390/polym15010230
Chicago/Turabian StyleMazlan, Nyak Syazwani Nyak, Kushairi Mohd Salleh, Mohamad Khalid Khairunnisa-Atiqah, Abdul Hair Ainul Hafiza, Marhaini Mostapha, Amanda V. Ellis, and Sarani Zakaria. 2023. "Macro-Size Regenerated Cellulose Fibre Embedded with Graphene Oxide with Antibacterial Properties" Polymers 15, no. 1: 230. https://doi.org/10.3390/polym15010230