Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Equipment and Methods
3. Results and Discussions
3.1. Synthesis and Structural Characterization
3.2. Morphology
3.3. The Hydrogel Stability over Time
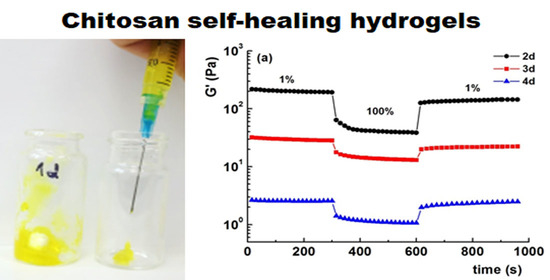
3.4. Visual Testing of Self-Healing Ability of the Hydrogels
3.5. Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Hsu, S. Synthesis and biomedical applications of self-healing hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Joshi, N.; Thorat, K.; Kaur, S.; Chandan, R.; Banerjee, R. A tumor responsive self healing prodrug hydrogel enables synergistic action of doxorubicin and miltefosine for focal combination chemotherapy. J. Mater. Chem. B 2019, 7, 2920–2925. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: From old chemistry to modern day innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-healing hydrogels: Preparation, mechanism and advancement in biomedical applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical applications. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Lehn, J.-M. Dynamers: Dynamic molecular and supramolecular polymers. Aust. J. Chem. 2010, 63, 611–623. [Google Scholar] [CrossRef]
- Yu, R.; Petit, E.; Barboiu, M.; Li, S.; Sun, W.; Chen, C. Biobased dynamic hydrogels by reversible imine bonding for controlled release of thymopentin. Mater. Sci. Eng. C 2021, 127, 112210. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Malik, U.S.; Niazi, M.B.K.; Jahan, Z.; Zafar, M.I.; Vo, D.-V.N.; Sher, F. Nano-structured dynamic Schiff base cues as robust self-healing polymers for biomedical and tissue engineering applications: A review. Environ. Chem. Lett. 2022, 20, 495–517. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Papadakis, C.M.; Kang, J.-J.; Lin, J.-M.; Hsu, S. Injectable phenolic-chitosan self-healing hydrogel with hierarchical micelle architectures and fast adhesiveness. Chem. Mater. 2021, 33, 3945–3958. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Chen, Y.M.; Zhang, P.; Zhang, Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci. Rep. 2016, 6, 37841. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 2018, 186, 54–63. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; St Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kari, F.W. A critical review of the toxicology of glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of glyoxal. J. Am. Coll. Toxicol. 1995, 14, 348–363. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a crosslinking method toward multifunctional hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Olaru, A.-M.; Marin, L.; Morariu, S.; Pricope, G.; Pinteala, M.; Tartau-Mititelu, L. Biocompatible chitosan based hydrogels for potential application in local tumour therapy. Carbohydr. Polym. 2018, 179, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Rosca, I.; Sandu, A.-I.; Marin, L. Chitosan crosslinking with a vanillin isomer toward self-healing hydrogels with antifungal activity. Int. J. Biol. Macromol. 2022, 205, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.-M.; Mititelu-Tartau, L.; Gavril, G.; Marin, L. Chitosan crosslinking with pyridoxal 5-phosphate vitamer toward biocompatible hydrogels for in vivo applications. Int. J. Biol. Macromol. 2021, 193, 1734–1743. [Google Scholar] [CrossRef]
- Lungu, R.; Paun, M.-A.; Peptanariu, D.; Ailincai, D.; Marin, L.; Nichita, M.-V.; Paun, V.-A.; Paun, V.-P. Biocompatible chitosan-based hydrogels for bioabsorbable wound dressings. Gels 2022, 8, 107. [Google Scholar] [CrossRef]
- Ailincai, D.; Rosca, I.; Morariu, S.; Mititelu-Tartau, L.; Marin, L. Iminoboronate-chitooligosaccharides hydrogels with strong antimicrobial activity for biomedical applications. Carbohydr. Polym. 2022, 276, 118727. [Google Scholar] [CrossRef] [PubMed]
- Bejan, A.; Doroftei, F.; Cheng, X.; Marin, L. Phenothiazine-chitosan based eco-adsorbents: A special design for mercury removal and fast naked eye detection. Int. J. Biol. Macromol. 2020, 162, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Natera, J.; Massad, W.; García, N.A. The role of vitamin B6 as an antioxidant in the presence of vitamin B2-photogenerated reactive oxygen species. A kinetic and mechanistic study. Photochem. Photobiol. Sci. 2012, 11, 938–945. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Grubbs, R.H. Aldehyde-Functionalized magnetic particles to capture off-target chemotherapeutic agents. ACS Omega 2020, 5, 29121–29126. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Ailincai, D.; Sandu, A.-I.; Marin, L. Amphiphilic chitosan-g-poly(trimethylene carbonate)—A new approach for biomaterials design. Int. J. Biol. Macromol. 2021, 193, 414–424. [Google Scholar] [CrossRef]
- Jarząbek, B.; Hajduk, B.; Domański, M.; Kaczmarczyk, B.; Nitschke, P.; Bednarski, H. Optical properties of phenylene–thiophene-based polyazomethine thin films. High Perform. Polym. 2017, 30, 1219–1228. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Anisiei, A.; Rosca, I.; Sandu, A.-I.; Bele, A.; Cheng, X.; Marin, L. Imination of microporous chitosan fibers—A route to biomaterials with “on demand” antimicrobial activity and biodegradation for wound dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoleru, E.; Dumitriu, R.P.; Ailiesei, G.-L.; Yilmaz, C.; Brebu, M. Synthesis of bioactive materials by in situ one-step direct loading of Syzygium aromaticum essential oil into chitosan-based hydrogels. Gels 2022, 8, 225. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Peritoneal carcinomatosis: Principles of management. Cancer Treat. Res. 1996, 82, 53–63. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Advances in brief cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar]
- Bratskaya, S.; Skatova, A.; Privar, Y.; Boroda, A.; Kantemirova, E.; Maiorova, M.; Pestov, A. Stimuli-responsive dual cross-linked N-carboxyethylchitosan hydrogels with tunable dissolution rate. Gels 2021, 7, 188. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Rosca, I.; Pinteala, M.; Mititelu-Tartau, L. Chitosan derivatives in macromolecular co-assembly nanogels with potential for biomedical applications. Biomacromolecules 2020, 21, 4231–4243. [Google Scholar] [CrossRef]
- Xiong, S.; Duan, L.; Cheng, X. Novel coumarin-chitosan fluorescent hydrogel for the selective identification of Fe2+ in aqueous systems. Polym. Chem. 2020, 11, 6066–6072. [Google Scholar] [CrossRef]
- Crugeiras, J.; Rios, A.; Riveiros, E.; Richard, J.P. Substituent effects on the thermodynamic stability of imines formed from glycine and aromatic aldehydes: Implications for the catalytic activity of pyridoxal-5′-phosphate. J. Am. Chem. Soc. 2009, 131, 15815–15824. [Google Scholar] [CrossRef] [Green Version]
- Ipate, A.M.; Serbezeanu, D.; Bargan, A.; Hamciuc, C.; Ochiuz, L.; Gherman, S. Poly(vinylpyrrolidone)-chitosan hydrogels as matrices for controlled drug release. Cellul. Chem. Technol. 2021, 55, 63–73. [Google Scholar] [CrossRef]
- Liang, Y.P.; Zhao, X.; Ma, P.X.; Guo, B.L.; Du, Y.P.; Han, X.Z. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Boddu, V.M.; Liu, S.X.; Liu, W.-C. A comparative study of microrheology of nanocellulose produced from corn stover using diffusing wave spectroscopy (DWS) and mechanical rheometry. Cellul. Chem. Technol. 2020, 54, 27–32. [Google Scholar] [CrossRef]
- Ruiz-Pardo, C.; Silva-Gutiérrez, L.; Lizardi-Mendoza, J.; López-Franco, Y.; Peniche-Covas, C.; Argüelles-Monal, W. Chitosan hydrogels based on the diels–alder click reaction: Rheological and kinetic study. Polymers 2022, 14, 1202. [Google Scholar] [CrossRef] [PubMed]
- Geisler, I.M.; Schneider, J.P. Evolution-based design of an injectable hydrogel. Adv. Funct. Mater. 2012, 22, 529–537. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Comparative evaluation of structured oil systems: Shellac oleogel, HPMC oleogel, and HIPE gel. Eur. J. Lipid Sci. Technol. 2015, 117, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhan, W.; Tang, X.; Mo, F.; Fu, L.; Lin, B. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P.; Tiwari, S. Dynamic imine bond based chitosan smart hydrogel with magnified mechanical strength for controlled drug delivery. Int. J. Biol. Macromol. 2020, 160, 489–495. [Google Scholar] [CrossRef]











| Sample | NH2/CHO Ratio | Chitosan (mg) | P5P (mg) | Water Volume (mL) | Water Content (%) | Solid Content (%) | Gelation Time |
|---|---|---|---|---|---|---|---|
| 1 | 1:1 | 60 | 79.7 | 4 | 96.63 | 3.37 | i |
| 1d | 1:1 | 60 | 79.7 | 8 | 98.28 | 1.72 | i |
| 1t | 1:1 | 60 | 79.7 | 12 | 98.85 | 1.15 | i |
| 1q | 1:1 | 60 | 79.7 | 16 | 99.13 | 0.87 | 5′ |
| 1.5 | 1.5:1 | 60 | 53.2 | 4 | 97.25 | 2.75 | i |
| 1.5d | 1.5:1 | 60 | 53.2 | 8 | 98.60 | 1.40 | i |
| 1.5t | 1.5:1 | 60 | 53.2 | 12 | 99.07 | 0.93 | i |
| 1.5q | 1.5:1 | 60 | 53.2 | 16 | 99.30 | 0.70 | 30′ |
| 2 | 2:1 | 60 | 39.8 | 4 | 97.57 | 2.43 | i |
| 2d | 2:1 | 60 | 39.8 | 8 | 98.77 | 1.23 | i |
| 2t | 2:1 | 60 | 39.8 | 12 | 99.18 | 0.82 | 5′ |
| 2q | 2:1 | 60 | 39.8 | 16 | 99.38 | 0.62 | 24 h |
| 2.5 | 2.5:1 | 60 | 31.8 | 4 | 97.76 | 2.24 | i |
| 2.5d | 2.5:1 | 60 | 31.8 | 8 | 98.87 | 1.13 | 5′ |
| 2.5t | 2.5:1 | 60 | 31.8 | 12 | 99.24 | 0.76 | 24 h |
| 2.5q | 2.5:1 | 60 | 31.8 | 16 | 99.43 | 0.57 | - |
| 3 | 3:1 | 60 | 26.6 | 4 | 97.88 | 2.12 | i |
| 3d | 3:1 | 60 | 26.6 | 8 | 98.93 | 1.07 | 30′ |
| 3t | 3:1 | 60 | 26.6 | 12 | 99.28 | 0.72 | 24 h |
| 3q | 3;1 | 60 | 26.6 | 16 | 99.46 | 0.54 | - |
| 3.5 | 3.5:1 | 60 | 22.8 | 4 | 97.97 | 2.03 | i |
| 3.5t | 3.5:1 | 60 | 22.8 | 12 | 99.31 | 0.69 | 24 h |
| 3.5q | 3.5:1 | 60 | 22.8 | 16 | 99.49 | 0.51 | - |
| 4 | 4:1 | 60 | 19.9 | 4 | 98.04 | 1.96 | i |
| 4d | 4:1 | 60 | 19.9 | 8 | 99.01 | 0.99 | 30′ |
| 4t | 4:1 | 60 | 19.9 | 12 | 99.34 | 0.66 | 24 h |
| 4q | 4:1 | 60 | 19.9 | 16 | 99.50 | 0.50 | - |
| 4.5 | 4.5:1 | 60 | 17.7 | 4 | 98.09 | 1.91 | i |
| 4.5t | 4.5:1 | 60 | 17.7 | 12 | 99.36 | 0.64 | - |
| 5 | 5:1 | 60 | 15.9 | 4 | 98.14 | 1.86 | i |
| 5d | 5:1 | 60 | 15.9 | 8 | 99.06 | 0.94 | 24 h |
| 6 | 6:1 | 60 | 13.3 | 4 | 98.20 | 1.80 | 5′ |
| 7 | 7:1 | 60 | 11.4 | 4 | 98.25 | 1.75 | 30′ |
| 8 | 8:1 | 60 | 10.0 | 4 | 98.28 | 1.72 | 5 h |
| 9 | 9:1 | 60 | 8.9 | 4 | 98.31 | 1.69 | 24 h |
| Sample | τl a (Pa) | γl a (%) | G′ b (Pa) | G″ b (Pa) | tan δ b (=G″/G′) | Structure Recovery c (%) |
|---|---|---|---|---|---|---|
| 1q | 6.5 | 39.7 | 15.7 | 1.9 | 0.12 | 80.1 |
| 2d | 40.8 | 15.8 | 326 | 28.7 | 0.09 | 74.7 |
| 2t | 7.4 | 18.9 | 38.4 | 4.8 | 0.13 | 79.1 |
| 2q | 4.7 | 25.2 | 20.2 | 2.8 | 0.14 | 80.0 |
| 2.5d | 20.4 | 15.8 | 87 | 7.3 | 0.08 | 76.1 |
| 2.5t | 0.4 | 6.3 | 16.3 | 2.1 | 0.13 | 89.3 |
| 3d | 11.7 | 15.8 | 77.4 | 6.3 | 0.08 | 78.8 |
| 3t | 0.3 | 9.9 | 1.1 | 0.4 | 0.36 | 94.7 |
| 3.5t | 2.1 | 39.7 | 3.3 | 0.5 | 0.15 | - |
| 4d | 0.6 | 25.1 | 3.4 | 0.9 | 0.26 | 97.6 |
| 4t | 0.2 | 25.3 | 0.4 | 0.2 | 0.50 | 100 |
| 6 | 5.9 | 46 | 5.6 | 2.1 | 0.38 | - |
| 9 | 8.8 | 39.7 | 11.6 | 2.1 | 0.18 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craciun, A.M.; Morariu, S.; Marin, L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers 2022, 14, 2570. https://doi.org/10.3390/polym14132570
Craciun AM, Morariu S, Marin L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers. 2022; 14(13):2570. https://doi.org/10.3390/polym14132570
Chicago/Turabian StyleCraciun, Anda Mihaela, Simona Morariu, and Luminita Marin. 2022. "Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization" Polymers 14, no. 13: 2570. https://doi.org/10.3390/polym14132570