Assessment of Antifungal Efficacy and Release Behavior of Fungicide-Loaded Chitosan-Carrageenan Nanoparticles against Phytopathogenic Fungi
Abstract
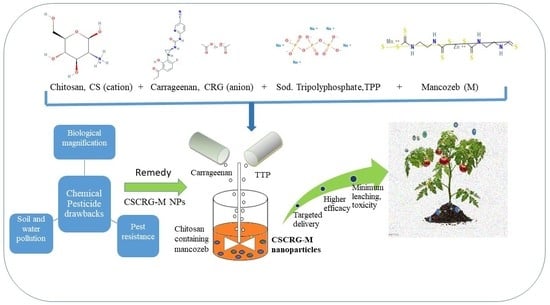
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Synthesis, Characterization and Optimization
2.1.1. Dynamic Light Scattering
2.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.3. X-ray Diffraction Spectroscopy (XRD)
2.1.4. Differential Scanning Calorimetry (DSC)
2.1.5. Thermogravimetric Analysis (TGA)
2.1.6. SEM/TEM
2.2. In Vitro Study
2.2.1. Antifungal Activity
2.2.2. Encapsulation Efficiency and Loading Capacity
2.2.3. Release Behavior
2.3. In Vivo Study
2.3.1. Bioefficacy of Nanoparticles in Pot House Conditions
2.3.2. Effect of Treatment on Germination Percentage
2.3.3. Effect of Treatment on Dry Weight per Plant
2.3.4. Effect of Treatment on the Root–Shoot Ratios of Plants
2.4. Cytotoxicity of Nanoformulations in Vero Cell Cultures
3. Materials and Methods
3.1. Reagents, Fungal Isolates and Plant Materials
3.2. Synthesis of Blank and Mancozeb-Loaded Chitosan-Carrageenan Conjugated Nanoparticles
3.3. Characterization
3.3.1. Size Optimization, Polydispersity Index and Zeta Potential
3.3.2. Fourier Transform Infra-Red (FTIR) Spectroscopy
3.3.3. Transmission Electron Microscopy (TEM)
3.3.4. Scanning Electron Microscopy (SEM)
3.3.5. X-ray Diffraction Spectroscopy (XRD)
3.3.6. Thermal Analysis Using Differential Scanning Calorimetry (DSC)
3.3.7. Thermogravimetric Analysis (TGA)
3.3.8. Encapsulation Efficiency (%) and Loading Capacity (%)
3.4. In Vitro Study
3.4.1. Antimicrobial Activity
3.4.2. Controlled Release Behavior
3.5. In Vivo Study
3.5.1. Bioefficacy of Nanofungicide against Target Fungi in Pot House Conditions
Treatment
Disease Assessment
Effects of Treatment on Plant Growth Parameters
3.6. Cytotoxicity of Nanoparticles in Vero Cells
- Briefly, Vero cell lines (derived from the kidney of an African green monkey) at a density of 1 × 104 per well were cultured in a 100 μL volume of cell culture medium (EMEM supplemented with 10% fetal bovine serum and antibiotics) in a 96-well cell culture plate.
- The cultured cells at 70–80% confluency were treated with different concentrations of nanoparticles (0.25–2000 μg/mL), well dispersed in 100 μL of deionized water with sonication and incubated in a CO2 incubator (at 37 °C with 5% CO2) for 24 h.
- After incubation, the samples were treated with 10 μL of the resazurin dye (1 mg/mL) prepared in EMEM media and incubated for 4 h under the same conditions.
- After 4 h, the pink-coloured resorufin is formed and absorbance was observed by a spectrophotometer (ELISA plate reader) at 590 nm.
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinutha, J.S.; Bhagat, D.; Bakthavatsalam, N. Nanotechnology in the management of polyphagous pest Helicoverpa armigera. J. Acad. Ind. Res. 2013, 1, 606–608. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R.; Dilbaghi, N. Development, and evaluation of alginate-chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef]
- Kalpana, S.; Rashmi, H.B.; Rao, N.H. Nanotechnology patents as R&D indicators for disease management strategies in agriculture. J. Intellect. Prop. Rights 2010, 15, 197–205. [Google Scholar]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.S. Nanotechnology in agriculture: Prospects and constraints. Nanotechnol. Sci. Appl. 2014, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Thakur, R.; Duhan, J.S.; Chaudhury, A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer arietinum). J. Chem. Technol. Biotechnol. 2018, 93, 3233–3243. [Google Scholar] [CrossRef]
- Cea, M.P.; Cartes, G.P.; Mora, M.L. Atrazine efficiency in an andisol as affected by clays and nanoclays in ethylcellulose controlled release formulations. Revista de la Ciencia del Suelo y Nutrición Vegetal 2010, 10, 62–77. [Google Scholar] [CrossRef] [Green Version]
- Salgueiro, A.M.; Daniel-da-Silva, A.L.; Fateixa, S.; Trindade, T. κ-Carrageenan hydrogel nanocomposites with release behavior mediated by morphological distinct Au nanofillers. Carbohydr. Polym. 2013, 91, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, G.; Li, J.; Feng, Y.; Peng, Y.; Zhao, X.; Tang, Y.; Zhang, Q. Stretchable hydrophobic modified alginate double-network nanocomposite hydrogels for sustained release of water-insoluble pesticides. J. Clean. Prod. 2019, 226, 122–132. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Barnela, M.; Chopra, M.; Manuja, A.; Chaudhury, A. Synthesis, characterization, and in vitro evaluation of cytotoxicity, and antimicrobial activity of chitosan–metal nanocomposites. J. Chem. Technol. Biotechnol. 2015, 90, 867–873. [Google Scholar] [CrossRef]
- Kaur, P.; Duhan, J.S.; Thakur, R. Comparative pot studies of chitosan and chitosan-metal nanocomposites as nano-agrochemicals against fusarium wilt of chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2018, 14, 466–471. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.S.; Pal, A.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef]
- Nörnberg, A.B.; Gehrke, V.R.; Mota, H.P.; Camargo, E.R.; Fajardo, A.R. Alginate-cellulose biopolymeric beads as efficient vehicles for encapsulation and slow-release of herbicide. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123970. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Azah Yusof, N.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Chitosan-based agronanofungicides as a sustainable alternative in the basal stem rot disease management. J. Agric. Food Chem. 2020, 68, 4305–4314. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Singh, B.P. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kumar, D.; Dilbaghi, N. Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 926–937. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Yang, Z.; Zhou, C.; Wang, C.; Li, S.; Ouyang, Q.; Yang, P. pH-responsive ultrasonic self-assembly spinosad-loaded nanomicelles and their antifungal activity to Fusarium oxysporum. React. Funct. Polym. 2019, 141, 123–132. [Google Scholar] [CrossRef]
- Borthakur, A.; Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G829–G838. [Google Scholar] [CrossRef] [PubMed]
- Fungicides Market Report—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2017–2025. Available online: https://www.prnewswire.com/news-releases/fungicides-market-mancozeb-chlorothalonil-metalaxyl-strobilurin-and-others-for-cereals--grains-oilseeds--pulses-fruits--vegetables-and-other-crops---global-industry-analysis-size-share-growth-trends-and-forecast-20-300243678.html (accessed on 6 October 2021).
- Cycoń, M.; Piotrowska-Seget, Z.; Kozdrój, J. Responses of indigenous microorganisms to a fungicidal mixture of mancozeb and dimethomorph added to sandy soils. Int. Biodeterior. Biodegrad. 2010, 64, 316–323. [Google Scholar] [CrossRef]
- Černohlávková, J.; Jarkovský, J.; Hofman, J. Effects of fungicides mancozeb and dinocap on carbon and nitrogen mineralization in soils. Ecotoxicol. Environ. Saf. 2009, 72, 80–85. [Google Scholar] [CrossRef]
- European Commission Regulation 2016/1 of 3 December 2015 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Bifenazate, Boscalid, Cyazofamid, Cyromazine, Dazomet, Dithiocarbamates, Fluazifop-p, Mepanipyrim, Metrafenone, Picloram, Propamocarb, Pyridaben, Pyriofenone, Sulfoxaflor, Tebuconazole, Tebufenpyrad and Thiram in or on Certain Products. Available online: https://eur-lex.europa.eu/eli/reg/2016/1/oj (accessed on 6 October 2021).
- Atuhaire, A.; Kaye, E.; Mutambuze, I.L.; Matthews, G.; Friedrich, T.; Jørs, E. Assessment of dithiocarbamate residues on tomatoes conventionally grown in Uganda and the effect of simple washing to reduce exposure risk to consumers. Environ. Health Insights 2017, 2, 1178630217712218. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Agency. The 2017 European Union report on pesticide residues in food. EFSA J. 2019, 17, e05743. [Google Scholar]
- Li, J.; Dong, C.; Yang, Q.; An, W.; Zheng, Z.; Jiao, B. Simultaneous determination of ethylenebisdithiocarbamate (ebdc) and propylenebisdithiocarbamate (pbdc) fungicides in vegetables, fruits, and mushrooms by ultra-high-performance liquid chromatography tandem mass spectrometry. Food Anal. Methods 2019, 12, 2045–2055. [Google Scholar] [CrossRef]
- Liu, J.; Legros, S.; Ma, G.; Veinot, J.G.; Von der Kammer, F.; Hofmann, T. Influence of surface functionalization, and particle size on the aggregation kinetics of engineered nanoparticles. Chemosphere 2012, 87, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorgan. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, Z.; Jeffery Daim, L.D. Preparation of chitosan–hexaconazole nanoparticles as fungicide nanodelivery system for combating Ganoderma disease in oil palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, S.; da Costa, A.M.R.; Grenha, A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of rice starch-ι-carrageenan biodegradable edible film: Effect of stearic acid on the film properties. Int. J. Biol. Macromol. 2016, 93, 952–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.; Liu, X.; Zhang, Y.; Yao, K. Study of phase behavior on chitosan/viscose rayon blend film. J. Appl. Polym. Sci. 1998, 67, 1965–1972. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using Response Surface Methodology. Int. J. Biol. Macromol. 2019, 143, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, R.O.; Patil, S.D. Fabrication and statistical optimization of starch-κ-carrageenan cross-linked hydrogel composite for extended release pellets of zaltoprofen. Int. J. Biol. Macromol. 2018, 120, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and characterization of pulullan-chitosan and pullulan-carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Zhao, C.; Xu, Y.; Lu, J.; Xiang, S.; Zong, F.; Wu, X. Polymeric nanoparticles as a metolachlor carrier: Water-based formulation for hydrophobic pesticides and absorption by plants. J. Agric. Food Chem. 2017, 65, 7371–7378. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Seman, I.A.; Hilmi, N.H.Z.; Daim, L.D.J. Enhanced fungicidal efficacy on Ganoderma boninense by simultaneous co-delivery of hexaconazole and dazomet from their chitosan nanoparticles. RSC Adv. 2019, 9, 27083–27095. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.A.; Aminabhavi, T.M. Development of novel interpenetrating network gellan gum-poly (vinyl alcohol) hydrogel microspheres for the controlled release of carvedilol. Drug Dev. Ind. Pharm. 2005, 31, 491–503. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Murugan, I.; Selvaraj, M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr. Polym. 2019, 212, 169–177. [Google Scholar] [CrossRef]
- Deng, L.; Qi, H.; Yao, C.; Feng, M.; Dong, A. Investigation on the properties of methoxy poly (ethylene glycol)/chitosan graft co-polymers. J. Biomater. Sci. Polym. Ed. 2007, 18, 1575–1589. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.; Abd El-Hai, F.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Boufas, S.; Benhamza, M.E.H.; Seghir, B.B.; Hadria, F. Synthesis and characterization of chitosan/carrageenan/hydroxyethyl cellulose blended gels. Asian J. Res. Chem. 2020, 13, 209–215. [Google Scholar] [CrossRef]
- Giroud, N.; Dorge, S.; Trouvé, G. Mechanism of thermal decomposition of a pesticide for safety concerns: Case of Mancozeb. J. Hazard. Mater. 2010, 184, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.Y.; Ouyang, X.K. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef]
- Long, J.; Yu, X.; Xu, E.; Wu, Z.; Xu, X.; Jin, Z.; Jiao, A. In situ synthesis of new magnetite chitosan/carrageenan nanocomposites by electrostatic interactions for protein delivery applications. Carbohydr. Polym. 2015, 131, 98–107. [Google Scholar] [CrossRef]
- Piyakulawat, P.; Praphairaksit, N.; Chantarasiri, N.; Muangsin, N. Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS Pharm. Sci. Technol. 2007, 8, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Zhao, W.T.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.C.; Li, Q. Morphology and thermal properties of calcium alginate/reduced graphene oxide composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.W.; Chun, S.C.; Chandrasekaran, M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Campos, E.V.R.; de Oliveira, J.L.; da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Fraceto, L.F. Polymeric and solid lipid nanoparticles for sustained release of carbendazim and tebuconazole in agricultural applications. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, P.; Kaur, P.; Kumar, A.; Duhan, J.S. Microwave assisted quick synthesis method of silver nanoparticles using citrus hybrid “Kinnow” and its potential against early blight of tomato. Res. Crop. 2017, 18, 650–655. [Google Scholar] [CrossRef]
- Bansal, P.; Kaur, P.; Surekha; Kumar, A.; Duhan, J.S. Biogenesis of silver nanoparticles using Aspergillus terreus, its cytotoxicity and potential as therapeutic against human pathogens. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 898–906. [Google Scholar]
- Bansal, P.; Kaur, P.; Duhan, J.S. Biogenesis of silver nanoparticles using Fusarium pallidoroseum and its potential against human pathogens. Ann. Biol. 2017, 33, 180–185. [Google Scholar]
- Kumar, N.; Salar, R.K.; Prasad, M.; Ranjan, K. Synthesis, characterization and anticancer activity of vincristine loaded folic acid-chitosan conjugated nanoparticles on NCI-H460 non-small cell lung cancer cell line. Egypt. J. Basic Appl. Sci. 2018, 5, 87–99. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, K.; Li, L.; Zhao, F.; Wang, Y.; Wang, M.; Shen, Y.; Cui, H.; Liu, D.; Guo, X. Spherical, and spindle-like abamectin-loaded nanoparticles by flash nanoprecipitation for southern root-knot nematode control: Preparation, and characterization. Nanomaterials 2018, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 2019, 18, 101079. [Google Scholar] [CrossRef]
- Macedo, D.F.; Dourado, S.M., Jr.; Nunes, E.S.; Marques, R.P.; Moreto, J.A. Controlled release of TBH herbicide encapsulated on Ca-ALG microparticles: Leaching and phytointoxication plants. Planta Daninha 2019, 37, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Rychter, P. Chitosan/glyphosate formulation as a potential, environmental friendly herbicide with prolonged activity. J. Environ. Sci. Health B 2019, 54, 681–692. [Google Scholar] [CrossRef]
- Xu, L.; Cao, L.D.; Li, F.M.; Wang, X.J.; Huang, Q.L. Utilization of chitosan-lactide copolymer nanoparticles as controlled release pesticide carrier for pyraclostrobin against Colletotrichum gossypii Southw. J. Dispers. Sci. Technol. 2014, 35, 544–550. [Google Scholar] [CrossRef]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidipour, M.; Maleki, A.; Kordi, S.; Birjandi, M.; Pajouhi, N.; Mohammadi, E.; Davari, B. Pectin/chitosan/tripolyphosphate nanoparticles: Efficient carriers for reducing soil sorption, cytotoxicity, and mutagenicity of paraquat and enhancing its herbicide activity. J. Agric. Food Chem. 2019, 67, 5736–5745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Y.; Zhao, L.; Han, L.; Lu, Y.; Hou, P.; Lu, J. Mitogen-activated protein kinase p38 and retinoblastoma protein signalling is required for DNA damage-mediated formation of senescence-associated heterochromatic foci in tumour cells. FEBS J. 2013, 280, 4625–4639. [Google Scholar] [CrossRef]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant assisted nisin loaded chitosan-carageenan nanocapsule synthesis for controlling food pathogens. Food Control 2014, 37, 158–164. [Google Scholar] [CrossRef]











| Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 |
|---|---|---|---|---|---|
| Concentrations | Particle Size (nm) | Zeta Potential (mV) | |||
| A: Chitosan (w/v) | B: Carrageenan (w/v) | C: Mancozeb (w/v) | |||
| 1. | 1 | 1 | 0.5 | 181.76 | −10.92 |
| 2. | 1.5 | 1.5 | 1.5 | 258.5 | −12.2 |
| 3. | 0.5 | 0.5 | 0.5 | 220.2 | −10.7 |
| 4. | 0.5 | 1 | 1 | 66.6 | −9.87 |
| 5. | 1 | 1.5 | 1 | 332.4 | −10.7 |
| 6. | 1.5 | 0.5 | 1.5 | 346.2 | −9.06 |
| 7. | 0.5 | 1.5 | 1.5 | 338.7 | −7.59 |
| 8. | 1.5 | 1.5 | 0.5 | 231.8 | 13.9 |
| 9. | 0.5 | 0.5 | 1.5 | 261 | 14.9 |
| 10. | 1 | 1 | 1 | 202.4 | 16 |
| 11. | 1 | 1 | 1.5 | 413.3 | 7.97 |
| 12. | 1 | 1 | 1 | 434.4 | 6.86 |
| 13. | 1.5 | 1 | 1 | 305.6 | 11.5 |
| 14. | 1 | 0.5 | 1 | 359.9 | 6.39 |
| 15. | 1.5 | 0.5 | 0.5 | 590.8 | 13.3 |
| 16. | 1 | 1 | 1 | 436.5 | 12.6 |
| 17. | 0.5 | 1.5 | 0.5 | 105.6 | −7.59 |
| Freshly Prepared Nanoparticles | |||
|---|---|---|---|
| Nanoparticles | Size | Zeta Potential | PDI |
| Blank Chitosan-Carrageenan nanocomposite (CSCRG-B) | 66.6 ± 7.5 | −12.2 ± 1.2 | 0.553 ± 0.1 |
| Mancozeb (1.0 mg/mL)-loaded Chitosan-Carrageenan nanocomposite (CSCRG-1.0) | 231.8 ± 0.9 | 13.9 ± 2.4 | 1 ± 0.2 |
| Storage stability of CSCRG NPs at 4 °C after 20 days | |||
| Blank Chitosan-Carrageenan nanocomposite (CSCRG-B) | 252.0 ± 2.1 | 18.1 ± 2.8 | 0.562 ± 0.2 |
| Mancozeb (1.0 mg/mL)-loaded Chitosan-Carrageenan nanocomposite (CSCRG-1.0) | 305.6 ± 0.7 | 7.97 ± 1.4 | 1 ± 0.5 |
| Phytopathogenic Fungus | Treatment | CSCRG NPs Fungus Diameter (mm) | CSCRG NPs % Inhibition = dc − dt/dc × 100 | Mancozeb Fungus Diameter (mm) | Mancozeb % Inhibition = dc − dt/dc × 100 |
|---|---|---|---|---|---|
| A. alternata (ITCC3640) | Blank NPs N 1.0 (1.0 ppm) | 12.5 ± 0.7 | 83.9 ± 0.7 b | -- | -- |
| Loaded NPs NF 0.5 (0.5 ppm) | 19.5 ± 0.7 | 74.8 ± 0.7 bc | 12 ± 1.4 | 84.5 ± 1.4 b | |
| Loaded NPs NF 1.0 (1.0 ppm) | 14 ± 0 | 81.9 ± 0 b | 11.5 ± 0.7 | 85.2 ± 0.7 b | |
| Loaded NPs NF 1.5 (1.5 ppm) | 13.5 ± 0.7 | 82.6 ± 0.7 b | 10.5 ± 0.7 | 86.5 ± 0.7 b | |
| S. lycopersici (ITCC5431) | Blank NPs N 1.0 (1.0 ppm) | 12.5 ± 0.7 | 62.1 ± 0.7 c | -- | -- |
| Loaded NPs NF 0.5 (0.5 ppm) | 14.5 ± 0.7 | 56.1 ± 0.7 c | 14.5 ± 0.7 | 56.1 ± 0.7 c | |
| Loaded NPs NF 1.0 (1.0 ppm) | 0 ± 0 | 100 ± 0 a | 0 ± 0 | 100.0 a | |
| Loaded NPs NF 1.5 (1.5 ppm) | 0 ± 0 | 100 ± 0 a | 0 ± 0 | 100.0 a | |
| Alternaria solani ITCC-3640 | Blank NPs N 1.0 (1.0 ppm) | 32.5 ± 3.5 | 50 ± 3.5 | -- | -- |
| Loaded NPs NF 0.5 (0.5 ppm) | 21 ± 1.4 | 67.7 ± 1.4 bc | 10.5 ± 0.7 | 83.8 ± 0.7 b | |
| Loaded NPs NF 1.0 (1.0 ppm) | 21 ± 0 | 67.7 ± 0 bc | 10 ± 0 | 84.6 ± 0 b | |
| Loaded NPs NF 1.5 (1.5 ppm) | 11 ± 0 | 83.1 ± 0 b | 10 ± 0 | 84.6 ± 0 b | |
| Sclerotonia sclerotiorum ITCC-5492 | Blank NPs N 1.0 (1.0 ppm) | 13.5 ± 0.7 | 60.3 ± 0.7 c | -- | -- |
| Loaded NPs NF 0.5 (0.5 ppm) | 15.5 ± 0.7 | 54.4 ± 0.7 c | 10.5 ± 0.7 | 69.1 ± 0.7 bc | |
| Loaded NPs NF 1.0 (1.0 ppm) | 0 ± 0 | 100 ± 0 a | 0 ± 0 | 100 ± 0 a | |
| Loaded NPs NF 1.5 (1.5 ppm) | 0 ± 0 | 100 ± 0 a | 0 ± 0 | 100 ± 0 a |
| Formulation | Encapsulation Efficiency (%) | Loading Capacity (%) |
|---|---|---|
| CSCRG-0.5 | 17.0 ± 1.20 | 87.3 ± 0.20 |
| CSCRG-1.0 | 38.1 ± 0.56 | 95.5 ± 1.15 |
| CSCRG-1.5 | 58.3 ± 0.83 | 92.9 ± 0.95 |
| Treatment | A. alternata | S. lycopersici | A. solani | S. sclerotiorum | ||||
|---|---|---|---|---|---|---|---|---|
| % DS | % DCE | % DS | % DCE | % DS | % DCE | % DS | % DCE | |
| Pure control C | 6.1 ± 1.4 | -- | 12.7 ± 3.5 | -- | 4.5 ± 0.7 | -- | 13.5 ± 2.1 | -- |
| Control CP | 42.9 ± 3.3 | -- | 40.9 ± 0.8 | -- | 29.4 ± 1.6 | -- | 27.4 ± 1.6 | -- |
| Fungicide F | 20.8 ± 6.9 | 76.6 ± 5.8 a | 20.2 ± 2.8 | 69.5 ± 1.8 a | 6.7 ± 0.6 | 76 ± 1.1 a | 9.9 ± 1 | 68 ± 6.9 a |
| Fungicide FP | 27.6 ± 3.4 | 72.8 ± 3.5 a | 12.9 ± 3.3 | 65.9 ± 1.1 a | 9.9 ± 0.5 | 65 ± 2.2 b | 10.9 ± 2.4 | 68.3 ± 3.4 a |
| Blank NPs N | 24.6 ± 5.7 | 66.4 ± 5.5 b | 12.2 ± 4.3 | 68.2 ± 4.2 a | 7.4 ± 1.6 | 78.1 ± 2.2 a | 10.9 ± 0.4 | 61.9 ± 5.7 b |
| Blank NPs NP | 22 ± 0.7 | 60.6 ± 3.4 | 19.4 ± 8.3 | 56.8 ± 0.5 b | 11.4 ± 1.3 | 66.1 ± 0.2 | 10 ± 0.6 | 61.4 ± 0.7 b |
| Loaded NPs NF | 10.4 ± 2.7 | 70.3 ± 6.9 a | 12.6 ± 4.6 | 73 ± 3.6 a | 6.4 ± 0.6 | 79.4 ± 1.7 a | 6.1 ± 0.2 | 77.2 ± 2.7 a |
| Loaded NPs NFP | 17.4 ± 3 | 68.4 ± 9.8 a | 12.8 ± 4 | 68.9 ± 0.9 b | 9.6 ± 2.8 | 75.8 ± 1 a | 7 ± 0.8 | 72.2 ± 3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Najda, A.; Duhan, J.S.; Kumar, B.; Chawla, P.; Klepacka, J.; Malawski, S.; Kumar Sadh, P.; Poonia, A.K. Assessment of Antifungal Efficacy and Release Behavior of Fungicide-Loaded Chitosan-Carrageenan Nanoparticles against Phytopathogenic Fungi. Polymers 2022, 14, 41. https://doi.org/10.3390/polym14010041
Kumar R, Najda A, Duhan JS, Kumar B, Chawla P, Klepacka J, Malawski S, Kumar Sadh P, Poonia AK. Assessment of Antifungal Efficacy and Release Behavior of Fungicide-Loaded Chitosan-Carrageenan Nanoparticles against Phytopathogenic Fungi. Polymers. 2022; 14(1):41. https://doi.org/10.3390/polym14010041
Chicago/Turabian StyleKumar, Ravinder, Agnieszka Najda, Joginder Singh Duhan, Balvinder Kumar, Prince Chawla, Joanna Klepacka, Seweryn Malawski, Pardeep Kumar Sadh, and Anil Kumar Poonia. 2022. "Assessment of Antifungal Efficacy and Release Behavior of Fungicide-Loaded Chitosan-Carrageenan Nanoparticles against Phytopathogenic Fungi" Polymers 14, no. 1: 41. https://doi.org/10.3390/polym14010041