Antimony Removal from Water by a Chitosan-Iron(III)[ChiFer(III)] Biocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Composite Beads
2.3. Characterization
2.4. pH Study
2.5. Effect of Initial Concentration
2.6. Kinetics
2.7. Ion Competition
2.8. Reusability Assessment
3. Results and Discussion
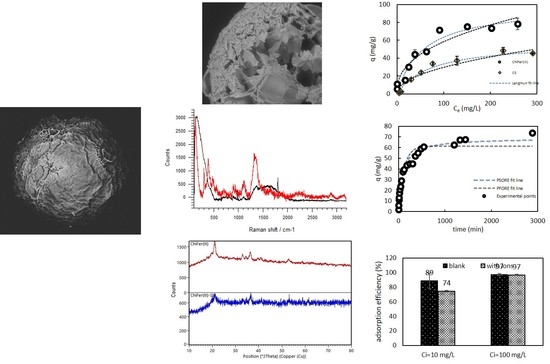
3.1. Characterization of the Material
Raman, XRD Spectroscopy, and SEM Observations
3.2. Effect of the pH
3.3. Effect of the Initial Concentration
3.4. Kinetics
3.5. Ion Competition
3.6. Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | neat chitosan powder |
| ChiFer(III) | chitosan/iron(III) hydroxide beads |
References
- Matusiewicz, H.; Krawczyk, M. Determination of total antimony and inorganic antimony species by hydride generation in situ trapping flame atomic absorption spectrometry: A new way to (ultra)trace speciation analysis. J. Anal. At. Spectrom. 2008, 23, 43–53. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substances and Disease Registry). Draft Toxicological Profile for Antimony and Compounds; ATSDR: Atlanta, GA, USA, 2017.
- Serfor-Armah, Y.; Nyarko, B.J.B.; Dampare, S.B.; Adomako, D. Levels of arsenic and antimony in water and sediment from Prestea, a gold mining town in Ghana and its environs. Water. Air. Soil Pollut. 2006, 175, 181–192. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017; ISBN 9789241549950. [Google Scholar]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation–flocculation–sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, F.; Pan, X.; Guo, J.; Wen, D. Removal of antimony from antimony mine flotation wastewater by electrocoagulation electrocoagulation with aluminum electrodes. J. Environ. Sci. 2011, 23, 1066–1071. [Google Scholar] [CrossRef]
- Saito, T.; Tsuneda, S.; Hirata, A.; Nishiyama, S.; Saito, K.; Saito, K.; Sugita, K.; Uezu, K.; Tamada, M.; Sugo, T. Removal of Antimony (III) Using Polyol-Ligand-Containing Porous Hollow-Fiber Membranes. Sep. Sci. Technol. 2004, 39, 3011–3022. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xiong, T.; Xu, A.; Pan, B.; Tang, K. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polymers 2018, 10, 835. [Google Scholar] [CrossRef]
- Ruiz, M.; Roset, L.; Demey, H.; Castro, S.; Sastre, A.M.; Pérez, J.J. Equilibrium and dynamic studies for adsorption of boron on calcium alginate gel beads using principal component analysis (PCA) and partial least squares (PLS). Materwiss. Werksttech. 2013, 44, 410–415. [Google Scholar] [CrossRef]
- Demey, H.; Tria, S.A.; Soleri, R.; Guiseppi-Elie, A.; Bazin, I. Sorption of his-tagged Protein G and Protein G onto chitosan/divalent metal ion sorbent used for detection of microcystin-LR. Environ. Sci. Pollut. Res. 2017, 24, 15–24. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bharskarapillai, A.; Velmurugan, S. Nano-titania-crosslinked chitosan composite as a superior sorbent for antimony (III) and (V). Carbohydr. Polym. 2014, 108, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Tuzen, M.; Kocal, İ. Application of chitosan-modified pumice for antimony adsorption from aqueous solution. Environ. Prog. Sustain. Energy 2017, 36, 1587–1596. [Google Scholar] [CrossRef]
- Deng, R.-J.; Jin, C.-S.; Ren, B.-Z.; Hou, B.-L.; Hursthouse, A.; Deng, R.-J.; Jin, C.-S.; Ren, B.-Z.; Hou, B.-L.; Hursthouse, A.S. The potential for the treatment of antimony-containing wastewater by iron-based adsorbents. Water 2017, 9, 794. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, R.; Zhao, X.; Qu, J. The mechanism of antimony(III) removal and its reactions on the surfaces of Fe-Mn Binary Oxide. J. Colloid Interface Sci. 2011, 363, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Vincent, T.; Ruiz, M.; Nogueras, M.; Sastre, A.M.; Guibal, E. Boron recovery from seawater with a new low-cost adsorbent material. Chem. Eng. J. 2014, 254, 463–471. [Google Scholar] [CrossRef]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A.M. Sorption of Hg (II) and Pb (II) Ions on Chitosan- Iron (III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A.M. Neodymium recovery by chitosan/iron(III) hydroxide [ChiFer(III)] sorbent material: Batch and column systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef]
- Ruiz, M.; Sastre, A.M.; Zikan, M.C.; Guibal, E. Palladium sorption on glutaraldehyde-crosslinked chitosan in fixed-bed systems. J. Appl. Polym. Sci. 2001, 81, 153–165. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 5, 385–471. [Google Scholar]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Sipos, P.; Berkesi, O.; Tombácz, E.; Pierre, T.G.; Webb, J. Formation of spherical iron(III) oxyhydroxide nanoparticles sterically stabilized by chitosan in aqueous solutions. J. Inorg. Biochem. 2003, 95, 55–63. [Google Scholar] [CrossRef]
- Nagai, T.; Kagi, H.; Yamanaka, T. Variation of hydrogen bonded O…O distances in goethite at high pressure. Am. Mineral. 2003, 88, 1423–1427. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Xi, J.; He, M.; Wang, K.; Zhang, G. Adsorption of antimony(III) on goethite in the presence of competitive anions. J. Geochem. Explor. 2013, 132, 201–208. [Google Scholar] [CrossRef]
- Bacelo, H.; Vieira, B.R.C.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Recovery and valorization of tannins from a forest waste as an adsorbent for antimony uptake. J. Clean. Prod. 2018, 198, 1324–1335. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams; National Institute of Advanced Industrial Science and Technology: Tokyo, Japan, 2005. [Google Scholar]
- Tella, M.; Pokrovski, G.S. Antimony(III) complexing with O-bearing organic ligands in aqueous solution: An X-ray absorption fine structure spectroscopy and solubility study. Geochim. Cosmochim. Acta 2009, 73, 268–290. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420051896. [Google Scholar]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Demey, H.; Melkior, T.; Chatroux, A.; Attar, K.; Thiery, S.; Miller, H.; Grateau, M.; Sastre, A.M.; Marchand, M. Evaluation of torrefied poplar-biomass as a low-cost sorbent for lead and terbium removal from aqueous solutions and energy co-generation. Chem. Eng. J. 2019, 361, 839–852. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Balasubramanian, R. Antimonite removal using marine algal species. Ind. Eng. Chem. Res. 2011, 50, 9864–9869. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bhaskarapillai, A.; Velmurugan, S. Enhancing the antimony sorption properties of nano titania-chitosan beads using epichlorohydrin as the crosslinker. J. Hazard. Mater. 2017, 334, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saeed, A.; Edyvean, R.G.J. Bioremoval of antimony(III) from contaminated water using several plant wastes: Optimization of batch and dynamic flow conditions for sorption by green bean husk (Vigna radiata). Chem. Eng. J. 2013, 225, 192–201. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Schiewer, S.; Cameron, R. Mechanistic elucidation and evaluation of biosorption of metal ions by grapefruit peel using FTIR spectroscopy, kinetics and isotherms modeling, cations displacement and EDX analysis. J. Chem. Technol. Biotechnol. 2009, 84, 1516–1526. [Google Scholar] [CrossRef]
- Valverde Armas, P.E.; Galarza Romero, B.A. Caracterización Geoquímica e Isotópica del Agua Superficial y Subterránea en el área de Influencia del río Siete y de las Actividades Mineras en el Distrito minero de Ponce Enríquez; Escuela Politécnica del Ejército: Sangolquí, Ecuador, 2012. [Google Scholar]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]







| Material | pH | T | qmax | Isotherm Fitting | Reference |
|---|---|---|---|---|---|
| (°C) | (mg/g) | ||||
| Sargassum sp. | 18.1 | [39] | |||
| Nano-titania chitosan—crosslinking with epichlorohydrin | 84.91 | [40] | |||
| Raw pumice | 5 | 20 | 44.80 | [14] | |
| Chitosan-modified pumice | 5 | 20 | 88.90 | [14] | |
| Green bean husk | 4 | 25 | 20.14 | [41] | |
| ChiFer(III) beads (present study) | 6 | room temperature | 98.68 | Langmuir | |
| Neat chitosan (present study) | 6 | room temperature | 57.99 | Langmuir |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapo, B.; Demey, H.; Carchi, T.; Sastre, A.M. Antimony Removal from Water by a Chitosan-Iron(III)[ChiFer(III)] Biocomposite. Polymers 2019, 11, 351. https://doi.org/10.3390/polym11020351
Lapo B, Demey H, Carchi T, Sastre AM. Antimony Removal from Water by a Chitosan-Iron(III)[ChiFer(III)] Biocomposite. Polymers. 2019; 11(2):351. https://doi.org/10.3390/polym11020351
Chicago/Turabian StyleLapo, Byron, Hary Demey, Tanya Carchi, and Ana María Sastre. 2019. "Antimony Removal from Water by a Chitosan-Iron(III)[ChiFer(III)] Biocomposite" Polymers 11, no. 2: 351. https://doi.org/10.3390/polym11020351