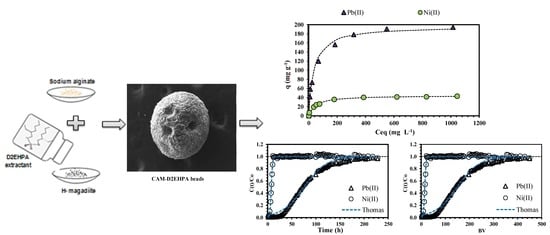
Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sorbents
2.2.1. Preparation of H-Magadiite/D2EHPA Material (HM-D2EHPA)
2.2.2. Preparation of the Calcium Alginate/H-Magadiite-D2EHPA Material (CAM-D2EHPA)
2.3. Characterization of Sorbents
2.4. Methods
2.4.1. Effect of Equilibrium pH
2.4.2. Sorption Isotherms
2.4.3. Effect of Contact Time
2.4.4. Sorption–Desorption Experiments
2.4.5. Dynamic System
3. Results and Discussion
3.1. Characterization of the Materials
3.1.1. FTIR Analyses
3.1.2. SEM–EDX Analyses
3.2. Effect of pH
3.3. Sorption Isotherms
3.4. Sorption Kinetics
3.5. Sorption–Desorption Cycles
3.6. Dynamic System
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| HM | H-magadiite |
| D2EHPA | Bis (2-ethylhexyl) phosphoric acid |
| HM-D2EHPA | H-magadiite impregnated with D2EHPA |
| CA | calcium alginate beads |
| CAM-D2EHPA | HM-D2EHPA immobilized into calcium alginate beads |
References
- Seyedi, S.M.; Anvaripour, B.; Motavassel, M.; Jadidi, N. Comparative Cadmium Adsorption from Water by Nanochitosan and Chitosan. Int. J. Eng. Innov. Technol. 2013, 2, 145–148. [Google Scholar]
- Gorchev, H.G.; Ozolins, G. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2011; Volume 1, p. 564. [Google Scholar]
- Mirbagheri, S.A.; Hosseini, S.N. Pilot plan investigation on petrochemical wastewater treatment for the removal of copper and chromium with the objective of reuse. Desalination 2005, 171, 85–93. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu+2, Cd+2 and Pb+2 adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Aji, B.A.; Yavuz, Y.; Koparal, A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar iron electrodes. Sep. Purif. Technol. 2012, 86, 248–254. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Coll, M.T.; Fortuny, A.; Kedari, C.S.; Sastre, A.M. Studies on the extraction of Co(II) and Ni(II) from aqueous chloride solutions using Primene JMT-Cyanex272 ionic liquid extractant. Hydrometallurgy 2012, 125–126, 24–28. [Google Scholar] [CrossRef]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid flotation-membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4046. [Google Scholar] [CrossRef]
- Samper, E.; Rodriguez, M.; De la Rubia, M.A.; Prats, D. Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep. Purif. Technol. 2009, 65, 337–342. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evaluation of chelate and cation exchange resins to remove copper ions. Powder Technol. 2016, 301, 520–525. [Google Scholar] [CrossRef]
- Kang, S.Y.; Lee, J.U.; Moon, S.H.; Kim, KW. Competitive adsorption characteristics of Co+2, Ni+2, and Cr+3 by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Guibal, E. Selective removal of Hg(II) from aqueous solution by functionalized magnetic-macromolecular hybrid material. Chem. Eng. J. 2015, 281, 345–359. [Google Scholar] [CrossRef]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A.M. Neodymium recovery by chitosan/Iron(III) hydroxide [ChiFer(III)] sorbent material: Batch and Column systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef]
- Demey, H.; Tria, S.A.; Soleri, R.; Guiseppi-Elie, A.; Bazin, I. Sorption of his-tagged Protein G and Protein G onto chitosan/divalent metal ion sorbent used for detection of microcystin-LR, Environ. Sci. Pollut. Res. Int. 2017, 24, 15–24. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Pereira, A.S.; Ferreira, G.; Caetano, L.; Castro, R.S.D.; dos Santos, A.; Padilha, P.M.; Castro, G.R. 4-amine-2-mercaptopyrimidine modified silica gel applied in Cd(II) and Pb(II) extraction from an aqueous medium. Pol. J. Chem. Technol. 2010, 12, 7–11. [Google Scholar] [CrossRef]
- Tzvetkova, P.; Nickolov, R. Modified and unmodified silica gel used for heavy metal ions removal from aqueous solutions. J. Univ. Chem. Technol. Metall. 2012, 47, 498–504. [Google Scholar]
- Bari, F.; Begum, N.; Jamaludin, S.B.; Hussin, K. Extraction and separation of Cu(II), Ni(II) and Zn(II) by sol–gel silica immobilized with Cyanex 272. Hydrometallurgy 2009, 96, 140–147. [Google Scholar] [CrossRef]
- Bouazza, D.; Miloudi, H.; Adjdir, M.; Tayeb, A.; Boos, A. Competitive adsorption of Cu (II) and Zn (II) on impregnate raw Algerian bentonite and efficiency of extraction. Appl. Clay Sci. 2018, 151, 118–123. [Google Scholar] [CrossRef]
- Ge, M.; Wang, X.; Du, M.; Liang, G.; Hu, G.; Jahangir-Alam, S.M. Adsorption analyses of phenol from aqueous solutions using magadiite modified with organo-functional groups: Kinetic and equilibrium studies. Materials 2019, 12, 96. [Google Scholar] [CrossRef]
- Ge, M.; Cao, L.; Du, M.; Hu, G.; Jahangir-Alam, S.M. Adsorptive characterization of a pure magadiite and an organic modified magadiite on removal of methylene blue from related aqueous solution. Mater. Chem. Phys. 2018, 217, 533–540. [Google Scholar] [CrossRef]
- Berdous, D.; Akretche, D. Recovery of heavy metal using solvent impregnated resin (SIR) coupled with Donnan Dialysis. Mater. Sci. Appl. 2012, 3, 704–712. [Google Scholar] [CrossRef]
- Vellaichamy, S. Adsorptive separation of copper, nickel, lead, zinc and cadmium from aqueous solution using MWCNTs impregnated with D2EHPA and prior to their determination by FAAS: Kinetic and equilibrium studies. Sep. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kozłowski, C.A.; Koziol, J.J. Transport of Zn (II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers. Sep. Purif. Technol. 2007, 57, 430–434. [Google Scholar] [CrossRef]
- Attar, K.; Bouazza, D.; Miloudi, H.; Tayeb, A.; Boos, A.; Sastre, A.M.; Demey-Cedeno, H. Cadmium removal by a low-cost magadiite-based material: Characterization and sorption applications. J. Environ. Chem. Eng. 2018, 6, 5351–5360. [Google Scholar] [CrossRef]
- Mousa, N.; Simonescu, C.M.; Pătescu, R.E.; Onose, C.; Tardei, C.; Culiţă, D.C.; Oprea, O.; Patroi, D.; Lavric, V. Pb2+ removal from aqueous synthetic solutions by calcium alginate and chitosan coated calcium alginate. React. Funct. Polym. 2016, 109, 137–150. [Google Scholar] [CrossRef]
- Ruiz, M.; Tobalina, C.; Demey-Cedeno, H.; Barron-Zambrano, J.A.; Sastre, A.M. Sorption of boron on calcium alginate gel beads. React. Funct. Polym. 2013, 73, 653–657. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013. [Google Scholar] [CrossRef]
- Demey-Cedeño, H.; Ruiz, M.; Barron-Zambrano, J.A.; Sastre, A.M. Boron removal from aqueous solutions using alginate gel beads in fixed-bed systems. J. Chem. Technol. Biotechnol. 2014, 89, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, M.; Roset, L.; Demey, H.; Castro, S.; Sastre, A.M.; Perez, J.J. Equilibrium and dynamic studies for adsorption of boron on calcium alginate beads using principal components analysis (PCA) and partial least squares (PLS). Materialwiss. Werkstofftech. 2013, 44, 410–415. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Gao, Y.; Zhang, S.; Wu, K. Adsorption of rare earths (III) using an efficient sodium alginate hydrogel cross-linked with poly-γ-Glutamate. PLoS ONE 2015, 10, e0124826. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. Adsorption properties toward trivalent rare earths by alginate beads doping with silica. Ind. Eng. Chem. Res. 2013, 52, 3453–3461. [Google Scholar] [CrossRef]
- Garlaschelli, F.; Alberti, G.; Fiol, N.; Villaescusa, I. Application of anodic stripping voltammetry to assess sorption performance of an industrial waste entrapped in alginate beads to remove As (V). Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Ngomsik, A.; Beea, A.; Siauguea, J.; Talbot, D.; Cabuila, V.; Coted, G. Co (II) removal by magnetic alginate beads containing Cyanex 272®. J. Hazard. Mater. 2009, 166, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Thirumavalavan, M.; Lee, J. Effective adsorption of heavy metal ions (Cu2+, Pb2+, Zn2+) from aqueous solution by immobilization of adsorbents on Ca-alginate beads. Toxicol. Environ. Chem. 2010, 92, 697–705. [Google Scholar] [CrossRef]
- Rojo, J.M.; Ruiz-Hitzky, E.; Sanz, J. Proton-sodium exchange in magadiite. Spectroscopic study (NMR, IR) of the evolution of interlayer OH groups. Inorg. Chem. 1988, 27, 2785–2790. [Google Scholar] [CrossRef]
- Song, S.; Gao, Q.; Jiang, J.; Gao, L.; Yu, L. A novel kind of porous carbon nitride using H-magadiite as the template. Mater. Lett. 2008, 62, 2520–2523. [Google Scholar] [CrossRef]
- Darvishi, D.; Haghshenas, D.F.; Keshavarz Alamdari, E.; Sadrnezhaad, S.K.; Halali, M. Synergistic effect of Cyanex 272 and Cyanex 302 on separation of cobalt and nickel by D2EHPA. Hydrometallurgy 2005, 77, 227–238. [Google Scholar] [CrossRef]
- Bidari, E.; Irannajad, M.; Gharabaghi, M. Investigation of the influence of acetate ions on cadmium extraction with D2EHPA. Hydrometallurgy 2014, 144–145, 129–132. [Google Scholar] [CrossRef]
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd (II), Cu (II) and Pb (II). Chem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Nasiruddin Khan, M.; Sarwar, A. Determination of points of zero charge of natural and treated adsorbents. Surf. Rev. Lett. 2007, 14, 461–469. [Google Scholar] [CrossRef]
- Haug, A. Dissociation of alginic acid. Acta Chem. Scand. 1961, 15, 950–952. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Bohli, T.; Villaescusa, I.; Ouederni, A. Comparative study of bivalent cationic metals adsorption Pb(II), Cd(II), Ni(II) and Cu(II) on olive stones chemically activated carbon. J. Chem. Eng. Process Technol. 2013, 4, 158–164. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorptive Accumulation of Cd(II), Co(II), Cu(II), Pb(II) and Ni(II) Ions from Water onto Kaolinite: Influence of Acid Activation. Adsorpt. Sci. Technol. 2009, 27, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.P.; Saucedo, I.; Navarro, R.; Avila, M.; Guibal, E. Selective Separation of Fe(III), Cd(II), and Ni(II) from Dilute Solutions Using Solvent-Impregnated Resins. Ind. Eng. Chem. Res. 2001, 40, 6004–6013. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef] [Green Version]
- Puppa, L.D.; Komarek, M.; Bordas, F.; Bollinger, J.C.; Joussein, E. Adsorption of copper, cadmium, lead and zinc onto a synthetic manganese oxide. J. Colloid Interface Sci. 2013, 399, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Abu Al-Rub, F.A.; El-Naas, M.H.; Benyahia, F.; Ashour, I. Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem. 2004, 39, 1767–1773. [Google Scholar] [CrossRef]
- Alba, J.; Navarro, R.; Saucedo, I.; Vincent, T.; Guibal, E. Extractant Immobilization in Alginate Capsules (Matrix- and Mononuclear-Type): Application to Pb(II) Sorption from HCl Solutions. Materials 2017, 10, 634. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A.M. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef]
- Ewecharoena, A.; Thiravetyana, P.; Wendelb, E.; Bertagnolli, H. Nickel adsorption by sodium polyacrylate-grafted activated carbon. J. Hazard. Mater. 2009, 171, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Melkior, T.; Chatroux, A.; Attar, K.; Thiery, S.; Miller, H.; Grateau, M.; Sastre, A.M.; Marchand, M. Evaluation of torrefied poplar-biomass as a low-cost sorbent for lead and terbium removal from aqueous solutions and energy co-generation. Chem. Eng. J. 2019, 361, 839–852. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Boujraf, S.; Taouda, H.; Zerrouq, F. Assessment of adsorption kinetics for removal potential of Crystal Violet dye from aqueous solutions using Moroccan pyrophyllite. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 23, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Sag, Y.; Kutsal, T. Recent trends in the biosorption of heavy metals: A review. Biotechnol. Bioprocess Eng. 2001, 6, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Kleinübing, S.J.; Da Silva, E.A.; Da Silva, M.G.C.; Guibal, E. Equilibrium of Cu(II) and Ni(II) biosorption by marine alga Sargassum filipendula in a dynamic system: Competitiveness and selectivity. Bioresour. Technol. 2011, 7, 4610–4617. [Google Scholar] [CrossRef]







| Initial pH | Equilibrium pH | SE Pb(II) (%) | SE Ni(II) (%) | DPb | DNi | |
|---|---|---|---|---|---|---|
| 1.27 | 1.64 | 87.2 | 0.6 | 6.8 | 0.01 | 1136.5 |
| 1.44 | 2.10 | 95.8 | 11.2 | 22.5 | 0.13 | 179.4 |
| 1.58 | 2.83 | 97.9 | 42.6 | 48.6 | 0.74 | 65.6 |
| 1.87 | 2.92 | 98.4 | 47.2 | 59.5 | 0.90 | 66.7 |
| 2.05 | 3.34 | 99.1 | 62.5 | 104.4 | 1.66 | 62.7 |
| Experimental | Langmuir | Freundlich | Sips | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal | qexp (mg g−1) | qmax (mg g−1) | kL (L mg−1) | r2 | kF (mg1−1/n g−1 L1/n) | nF | r2 | qmax (mg g−1) | kS (L mg−1) | nS | r2 |
| Pb(II) | 197.17 | 197.26 | 0.027 | 0.990 | 33.55 | 3.68 | 0.955 | 221.68 | 0.054 | 1.36 | 0.998 |
| Ni(II) | 42.84 | 44.36 | 0.022 | 0.992 | 9.20 | 4.31 | 0.957 | 47.73 | 0.050 | 1.32 | 0.996 |
| Sorbents | Experimental Conditions | Sorption Capacity (mg g−1) Pb(II) | Sorption Capacity (mg g−1) Ni(II) | References |
|---|---|---|---|---|
| Silica gel (SG) Modified silica gel (S2A) | pH = 2.0 | 0.72 - | 0.34 0.95 | [18] |
| Kaolinite Acid-activated kaolinite | pH = 7.0 | 11.10 12.10 | 10.40 11.90 | [47] |
| Mesoporous activated carbon adsorbent | pH = 7.0 | 20.32 | - | [49] |
| EIRs | pH = 1.0 | 80.00 | - | [52] |
| 4-amino-2-mercaptopyrimidine modified silica gel | pH = 3.0 | 80.20 | - | [17] |
| Fe3O4-SO3H MNPs | pH = 7.0 | 108.93 | - | [53] |
| ChiFer(III) | pH = 4.5 | 116.03 | - | [54] |
| Amorphous manganese oxide (AMO) | pH = 4.0 pH = 5.5 | 124.32 125.15 | - - | [50] |
| CAM-D2EHPA | pH = 4.0 | 197.26 | 44.36 | [This work] |
| AF-PEI A-PEI | pH = 4.0 | 225.85 229.99 | 60.45 48.71 | [16] |
| XAD-2-Cyanex 272 XAD-2-Cyanex 302 | pH = 2.0 | - | 6.49 3.65 | [48] |
| Free dead algal cells Blank alginate beads Immobilized dead algal cells | pH = 5.0 | - - - | 13.90 25.60 31.30 | [51] |
| Activated carbon Irradiation grafted Activated carbon | pH = 7.0 | - - | 44.10 55.70 | [55] |
| Raw poplar (trunk) Torrefied poplar (250 °C, 75 min) Torrefied poplar (280 °C, 60 min) | pH = 4.0 pH = 4.0 pH = 4.0 | 28.50 27.55 30.89 | - - - | [56] |
| Experimental | Pseudo-First Order Model (PFORE) | Pseudo-Second Order Model (PSORE) | |||||
|---|---|---|---|---|---|---|---|
| Metal | qexp (mg g−1) | k1 (min−1) | q1 (mg g−1) | r2 | k2 (g mg−1 min−1) | q1 (mg g−1) | r2 |
| Pb(II) | 4.71 | 0.057 | 4.57 | 0.992 | 0.019 | 4.88 | 0.994 |
| Ni(II) | 4.22 | 0.097 | 4.07 | 0.954 | 0.037 | 4.30 | 0.992 |
| Cycles | Pb(II) | Ni(II) | ||||
|---|---|---|---|---|---|---|
| Number of Cycles | pHe | Sorption (%) | Desorption (%) | pHe | Sorption (%) | Desorption (%) |
| 1 | 2.5 | 97.8 | 94.0 | 2.5 | 83.2 | 99.1 |
| 2 | 2.6 | 97.8 | 92.5 | 2.6 | 74.8 | 99.5 |
| 3 | 2.5 | 97.6 | 88.8 | 2.5 | 74.8 | 99.6 |
| 4 | 2.6 | 97.7 | 97.5 | 2.7 | 80.7 | 93.7 |
| 5 | 2.6 | 97.9 | 89.3 | 2.6 | 76.5 | 92.6 |
| 6 | 2.6 | 97.7 | 94.1 | 2.6 | 74.7 | 95.0 |
| 7 | 2.6 | 97.7 | 89.0 | 2.6 | 76.3 | 93.8 |
| 8 | 2.6 | 98.3 | 87.0 | 2.6 | 74.9 | 96.5 |
| 9 | 2.6 | 98.3 | 81.4 | 2.6 | 77.2 | 94.7 |
| 10 | 2.6 | 98.5 | 88.0 | 2.6 | 77.2 | 95.7 |
| Experimental | Thomas Parameters | |||||
|---|---|---|---|---|---|---|
| Sorbate | qexp (mg∙g−1) | qBP (mg∙g−1) | BVBP | qT (mg∙g−1) | KT (L∙h−1∙mg−1) | r2 |
| Pb(II) | 61.50 | 22.3 | 59.13 | 56.71 | 8.62 × 10−4 | 0.986 |
| Ni(II) | 5.51 | 0.58 | 5.91 | 5.69 | 1.44 × 10−2 | 0.992 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. https://doi.org/10.3390/polym11020340
Attar K, Demey H, Bouazza D, Sastre AM. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers. 2019; 11(2):340. https://doi.org/10.3390/polym11020340
Chicago/Turabian StyleAttar, Keltoum, Hary Demey, Djamila Bouazza, and Ana Maria Sastre. 2019. "Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials" Polymers 11, no. 2: 340. https://doi.org/10.3390/polym11020340