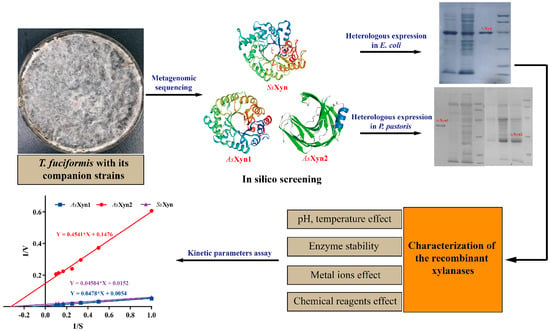
Mining, Identification, and Characterization of Three Xylanases from the Microbiota of T. fuciformis with Its Companion Strains
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Metagenomic Sequencing
2.2. In Silico Screening of Xylanase Condidates
2.3. Expression of the Xylanase Candidates in E. coli and P. pastoris
2.4. Characterization of the SsXyn Enzyme in E. coli
2.5. Characterization of the AsXyn1 and AsXyn2 Enzymes in P. pastoris
3. Materials and Methods
3.1. Strains and Plasmids
3.2. Metagenomic DNA Extraction and Analysis
3.3. Sequence Analysis
3.4. RNA Extraction and cDNA Amplification
3.5. Heterologous Expression of the Putative Xylanase Candidates in E. coli
3.6. Heterologous Expression of the Putative Xylanase Candidates in P. pastoris
3.7. Purification of Recombinant Enzymes
3.8. Enzyme Activity Assays
3.9. Characterization of Recombinant Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loqué, D.; Scheller, H.V.; Pauly, M. Engineering of plant cell walls for enhanced biofuel production. Curr. Opin. Plant Biol. 2015, 25, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, J.; Xiao, Z.; Shen, Q.; Zhang, R. Characterization and identification of the xylanolytic enzymes from Aspergillus fumigatus Z5. BMC Microbiol. 2015, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Bosetto, A.; Justo, P.I.; Zanardi, B.; Venzon, S.S.; Graciano, L.; dos Santos, E.L.; Simão Rde, C. Research Progress Concerning Fungal and Bacterial β-Xylosidases. Appl. Biochem. Biotechnol. 2016, 178, 766–795. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.R.; Filho, E.X. Insights into the mechanism of enzymatic hydrolysis of xylan. Appl. Microbiol. Biotechnol. 2016, 100, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Ariaeenejad, S.; Maleki, M.; Hosseini, E.; Kavousi, K.; Moosavi-Movahedi, A.A.; Salekdeh, G.H. Mining of camel rumen metagenome to identify novel alkali-thermostable xylanase capable of enhancing the recalcitrant lignocellulosic biomass conversion. Bioresour. Technol. 2019, 281, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.J.; Dong, L.; Zhang, Z.Y.; He, Y.; Ma, X. Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis. Food Sci. Hum. Well 2022, 11, 1010–1017. [Google Scholar] [CrossRef]
- Liu, D.M.; Zhu, H.Y.; Pujiana, D.; Zheng, L.S.; Chen, L.G.; Ma, A.M. Cloning and functional characterization of gpd and α-tubulin promoters from Annulohypoxylon stygium, a companion fungus of Tremella fuciformis. Mycoscience 2020, 61, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.; Hsiang, T.; Li, S.; Lin, L.; Wang, Q.; Chen, Q.; Xie, B.; Ming, R. Comparison of the mitochondrial genome sequences of six Annulohypoxylon stygium isolates suggests short fragment insertions as a potential factor leading to larger genomic size. Front. Microbiol. 2018, 9, 2079. [Google Scholar] [CrossRef]
- Robl, D.; Delabona Pda, S.; Mergel, C.M.; Rojas, J.D.; Costa Pdos, S.; Pimentel, I.C.; Vicente, V.A.; da Cruz Pradella, J.G.; Padilla, G. The capability of endophytic fungi for production of hemicellulases and related enzymes. BMC Biotechnol. 2013, 13, 94. [Google Scholar] [CrossRef]
- Wingfield, B.D.; Bills, G.F.; Dong, Y.; Huang, W.; Nel, W.J.; Swalarsk-Parry, B.S.; Vaghefi, N.; Wilken, P.M.; An, Z.; de Beer, Z.W.; et al. IMA Genome-F 9: Draft genome sequence of Annulohypoxylon stygium, Aspergillus mulundensis, Berkeleyomyces basicola (syn. Thielaviopsis basicola), Ceratocystis smalleyi, two Cercospora beticola strains, Coleophoma cylindrospora, Fusarium fracticaudum, Phialophora cf. hyalina, and Morchella septimelata. IMA Fungus 2018, 9, 199–223. [Google Scholar]
- Lin, H.; Sun, M.; Li, J.; Xu, Q.; Yang, B.; Wang, Q.; Xie, W.; Sun, S.; Hu, K.; Zhang, L. Purification and Characterization of Xylanase from Spent Mushroom Compost and its Application in Saccharification of Biomass Wastes. Bioresources 2018, 13, 220–230. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, G.; Li, A.; Chen, J.; Ma, L. Cloning and enzymatic characterization of a xylanase gene from a soil-derived metagenomics library with an efficient approach. Appl. Microbiol. Biotechnol. 2008, 80, 823. [Google Scholar] [CrossRef] [PubMed]
- Ariaeenejad, S.; Hosseini, E.; Maleki, M.; Kavousi, K.; Moosavi-Movahedi, A.A.; Salekdeh, G.H. Identification and characterization of a novel thermostable xylanase from camel rumen metagenome. Int. J. Biol. Macromol. 2019, 1, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Al-Darkazali, H.; Meevootisom, V.; Isarangkul, D.; Wiyakrutta, S. Gene expression and molecular characterization of a xylanase from chicken cecum metagenome. Int. J. Microbiol. 2017, 2017, 4018398. [Google Scholar] [CrossRef]
- Warnecke, F.; Luginbühl, P.; Ivanova, N.; Ghassemian, M.; Richardson, T.H.; Stege, J.T.; Cayouette, M.; McHardy, A.C.; Djordjevic, G.; Aboushadi, N. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 2007, 450, 560. [Google Scholar] [CrossRef]
- Li, R.; Kibblewhite, R.; Orts, W.J.; Lee, C.C. Molecular cloning and characterization of multidomain xylanase from manure library. World J. Microbiol. Biotechnol. 2009, 25, 2071–2078. [Google Scholar] [CrossRef]
- Cai, J.; Chen, X.L.; Fan, J.X.; Huang, X.M.; Li, R.; Sun, X.D.; Li, Q.Q.; Li, D.Y. Cloning and heterologous expression of a novel xylanase gene TAX1 from Trichoderma atroviride and its application in the deconstruction of corn stover. Appl. Biochem. Biotechnol. 2021, 193, 3029–3044. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Jiang, F.; Wu, J.; Xue, Y.; Gan, L.; Liu, J.; Long, M. Heterologous Expression and Characterization of an Acidic GH11 Family Xylanase from Hypocrea orientalis. Appl. Biochem. Biotechnol. 2018, 184, 228–238. [Google Scholar] [CrossRef]
- Li, G.; Zhou, X.; Li, Z.; Liu, Y.; Liu, D.; Miao, Y.; Wan, Q.; Zhang, R. Significantly improving the thermostability of a hyperthermophilic GH10 family xylanase XynAF1 by semi-rational design. Appl. Microbiol. Biotechnol. 2021, 105, 4561–4576. [Google Scholar] [CrossRef]
- Liew, K.J.; Ngooi, C.Y.; Shamsir, M.S.; Sani, R.K.; Chong, C.S.; Goh, K.M. Heterologous expression, purification and biochemical characterization of a new endo-1,4-β-xylanase from Rhodothermaceae bacterium RA. Protein Expres. Purif. 2019, 164, 105464. [Google Scholar] [CrossRef]
- Xu, B.; Dai, L.M.; Li, J.J.; Dent, M.; Miao, H.B.; Zhou, J.P.; Mu, Y.L.; Wu, Q.; Tang, X.H.; Yang, Y.J.; et al. Molecular and Biochemical Characterization of a Novel Xylanase from Massilia sp. RBM26 Isolated from the Feces of Rhinopithecus bieti. J. Microbiol. Biotechnol. 2015, 26, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Z.; Grosse, S.; Bergeron, H.; Lau, P.C.K. Cloning and characterization of the first GH10 and GH11 xylanases from Rhizopus oryzae. Appl. Microbiol. Biotechnol. 2014, 98, 8211–8222. [Google Scholar] [CrossRef] [PubMed]
- Shental-Bechor, D.; Levy, Y. Effect of glycosylation on protein folding. A close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Maldonado, R.; Vieira, D.S.; Alponti, J.S.; Bonneil, E.; Thibault, P.; Ward, R.J. Engineering the pattern of protein glycosylation modulates the thermostability of a GH11 xylanase. J. Biol. Chem. 2013, 288, 25522–25534. [Google Scholar] [CrossRef]
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard, W.A.; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 2012, 337, 975–980. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049. [Google Scholar] [CrossRef]







| Unigene | Name | Gene Annotation | Strain | Family | Gene Length | Identity |
|---|---|---|---|---|---|---|
| 11,018 | AsXyn1 | xylanase | A. stygium | GH10 | 1110 | 97% |
| 14,634 | AsXyn2 | xylanase | A. stygium | GH11 | 651 | 100% |
| 52,146 | SsXyn | xylanase | Sphingobacterium sp. | GH10 | 1149 | 95% |
| Metal Ions and Chemical Reagents | Relative Activity (%) | |||
|---|---|---|---|---|
| AsXyn1 | AsXyn2 | |||
| 1 mM | 10 mM | 1 mM | 10 mM | |
| Control | 100.0 ± 0.7 | 100.0 ± 0.9 | 100.0 ± 1.1 | 100.0 ± 0.9 |
| Cu2+ | 16 ± 1.6 | 2 ± 0.4 | 95 ± 2.0 | 41 ± 1.6 |
| Fe2+ | 54 ± 2.9 | 41 ± 0.4 | 76 ± 3.3 | 12 ± 1.3 |
| Fe3+ | 21 ± 1.8 | 4 ± 2.7 | 81 ± 2.2 | 46 ± 1.8 |
| Mn2+ | 75 ± 2.0 | 75 ± 2.0 | 68 ± 2.7 | 55 ± 1.8 |
| Co2+ | 110 ± 8.0 | 117 ± 4.4 | 89 ± 2.2 | 63 ± 1.8 |
| Zn2+ | 71 ± 0.4 | 40 ± 1.6 | 91 ± 1.1 | 60 ± 1.6 |
| Ca2+ | 111 ± 6.2 | 116 ± 3.3 | 94 ± 1.6 | 95 ± 1.8 |
| K+ | 101 ± 5.1 | 103 ± 7.3 | 94 ± 3.1 | 98 ± 1.1 |
| Ba2+ | 109 ± 3.3 | 130 ± 3.8 | 94 ± 2.0 | 96 ± 1.3 |
| Mg2+ | 111 ± 4.9 | 112 ± 4.7 | 97 ± 1.3 | 97 ± 1.3 |
| EDTA | 83 ± 3.1 | 64 ± 2.9 | 85 ± 3.6 | 69 ± 6.7 |
| SDS | 4 ± 1.3 | 2 ± 1.6 | 84 ± 1.3 | 2 ± 1.1 |
| Tween 80 | 115 ± 2.7 | 128 ± 14.4 | 95 ± 1.6 | 127 ± 5.3 |
| BME | 108 ± 1.6 | 80 ± 5.3 | 88 ± 1.3 | 78 ± 2.7 |
| Name | Primer Sequence (5′→3′) |
|---|---|
| 28a-AsXyn1-F | GGACAGCAAATGGGTCGCGGATCCGCCGACAGCATCGACGCCTT |
| 28a-AsXyn1-R | TGGTGCTCGAGTGCGGCCGCAAGCTTTTTCAGGGCGTTCACGACAG |
| 28a-Ssxyn-F | GGACAGCAAATGGGTCGCGGATCCAATATACAGGATCTGGAACA |
| 28a-Ssxyn-R | GTGGTGCTCGAGTGCGGCCGCAAGCTTCTTTTTTTTTTGAGTCAATG |
| 28a-AsXyn2-F | GGACAGCAAATGGGTCGCGGATCCTCGCCGCTCGACCTAATCAC |
| 28a-AsXyn2-R | TGCTCGAGTGCGGCCGCAAGCTTAGACTGCTCAACGGTAATC |
| 9k-AsXyn1-F | GCTGAAGCTTACGTAGAATTCCATCATCACCATCACCACGCCGACAGCATCGACGCCTT |
| 9k-AsXyn1-R | AGGCGAATTAATTCGCGGCCGCTTTCAGGGCGTTCACGACAG |
| 9k-AsXyn2-F | CTGAAGCTTACGTAGAATTCTCGCCGCTCGACCTAATCAC |
| 9k-AsXyn2-R | GCGAATTAATTCGCGGCCGCATGATGATGATGATGATGAGACTGCTCAACGGTAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Li, C.; Wei, C.; Lin, H.; Zhang, L. Mining, Identification, and Characterization of Three Xylanases from the Microbiota of T. fuciformis with Its Companion Strains. Catalysts 2024, 14, 15. https://doi.org/10.3390/catal14010015
Lin Y, Li C, Wei C, Lin H, Zhang L. Mining, Identification, and Characterization of Three Xylanases from the Microbiota of T. fuciformis with Its Companion Strains. Catalysts. 2024; 14(1):15. https://doi.org/10.3390/catal14010015
Chicago/Turabian StyleLin, Yanhuan, Changle Li, Chenxin Wei, Hui Lin, and Liaoyuan Zhang. 2024. "Mining, Identification, and Characterization of Three Xylanases from the Microbiota of T. fuciformis with Its Companion Strains" Catalysts 14, no. 1: 15. https://doi.org/10.3390/catal14010015