Study of the Composition and Properties of Bivalve Mollusk Shells as Promising Bio-Indifferent Materials for Photocatalytic Applications (Example of Practical Use)
Abstract
:1. Introduction
2. Results and Discussions
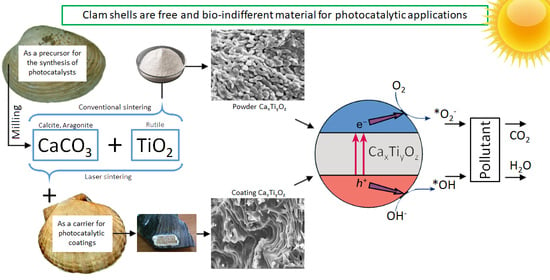
2.1. Options for Photocatalytic Applications
2.2. Characteristics Biological Items
2.3. Study of Composition and Properties
2.3.1. Phase and Elemental Composition
2.3.2. Organic Composition
2.3.3. “Powder-like” Morphology and Granulometric Properties
2.3.4. “Carrier-like” Mechanical and Functional Properties
2.3.5. Sorption Activity
2.3.6. Example of Practical Use
3. Materials and Methods
3.1. Description of Initial Substances
3.2. Description of the Analysis Methodologies
3.3. Place of Sampling Biological Items
3.4. Synthesis and Study of Photocatalyst Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, G.C.; Xing, L.S.; Hea, J.H.; Rong, L.; Hong, Z.G.; Anna, W.; Monika, W.; Agnieszka, G.P.; Bo, L.; Qiao, X.Y.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol. Environ. Saf. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Sami, U.K.; Ijaz, H. Impact of safe drinking water and clean fuels on health and wellbeing in Pakistan: A spatial analysis. Groundw. Sustain. Dev. 2021, 15, 100677. [Google Scholar] [CrossRef]
- Albert, S.; Laetitia, P.; François, P.; Sergi, G.S. Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef]
- Nafees, A.; Jerry, A.; Mohammad, Z.; Khan, S.S.; Xiao, J.Y.; Vijay, K.T.; Pablo, C.; Frederic, C. Visible light-conducting polymer nanocomposites as efficient photocatalysts for the treatment of organic pollutants in wastewater. J. Environ. Manag. 2021, 295, 113362. [Google Scholar] [CrossRef]
- Arunpandian, M.; Selvakumar, K.; Raja, A.; Rajasekaran, P.; Ramalingan, C.; Nagarajan, E.R.; Pandikumar, A.; Arunachalam, S. Rational design of novel ternary Sm2WO6/ZnO/GO nanocomposites: An affordable photocatalyst for the mitigation of carcinogenic organic pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124721. [Google Scholar] [CrossRef]
- Sumei, L.; Saisai, S.; Sha, C.; Hanbing, L.; Ziyi, L.; Yixuan, L.; Jiaying, F.; Linhua, X.; Jianrong, L. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967. [Google Scholar] [CrossRef]
- Menglu, Z.; Yu, Y.; Xiaoqiang, A.; Li-an, H. A critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Moses, G.P.; Elvera, L.V. WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water—A review. J. Water Process Eng. 2021, 40, 101930. [Google Scholar] [CrossRef]
- Nur Aqilah, M.R.; Wan Norharyati, W.S.; Farhana, A.; Lau, W.J.; Norhaniza, Y.; Ahmad, F.I. Review on tungsten trioxide as a photocatalysts for degradation of recalcitrant pollutants. J. Clean. Prod. 2021, 309, 127438. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Ramezanpoura, S.; Khatamian, M. Synthesis and characterization of thallium doped Mn3O4 as superior sunlight photocatalysts. J. Mol. Liq. 2017, 238, 248–253. [Google Scholar] [CrossRef]
- Mojgan, G.; Noshin, M.; Omid, A.; Masoud, S.N. Photocatalytic degradation of antibiotic using Ni-doped thallium(I) orthotungstate nanorods: Hydrothermal synthesis and characterization. J. Alloys Compd. 2021, 874, 159856. [Google Scholar] [CrossRef]
- Karunakaran, C.; Kalaivani, S. Enhanced visible light-photocatalysis by hydrothermally synthesized thallium-doped bismuth vanadate nanoparticles. Mater. Sci. Semicond. Process. 2014, 27, 352–361. [Google Scholar] [CrossRef]
- Elahe, R.; Mojgan, G.; Hassan, A.A.; Maryam, K.; Mahin, B.; Masoud, S.N. Simple preparation of chitosan-coated thallium lead iodide nanostructures as a new visible-light photocatalyst in decolorization of organic contamination. J. Mol. Liq. 2021, 341, 117299. [Google Scholar] [CrossRef]
- Renukadevi, R.; Sundaram, R.; Kaviyarasubc, K. Robust Hg0.023WO3 nanoparticles: Synthesis, characterization and application as relative humidity sensing material and photocatalyst for degradation of organic dye contamination. Nanosmatafrica 2021, 36, 192–198. [Google Scholar] [CrossRef]
- Datang, L.; Jiayin, L.; Jianting, T. Mercury oxide as an efficient photocatalyst for degradation of rhodamine B dye under visible-light irradiation. Solid State Sci. 2016, 61, 201–206. [Google Scholar] [CrossRef]
- Zhenghua, W.; Shiyu, Z.; Suping, Z.; Haibo, H. Synthesis of core–shell Fe3O4@SiO2@MS (M = Pb, Zn, and Hg) microspheres and their application as photocatalysts. J. Alloys Compd. 2011, 509, 6893–6898. [Google Scholar] [CrossRef]
- Yang, L.; Jian, Z.; Guangshan, Z.; Lilan, Z.; Shiyuan, D.; Enxiang, S.; Xinghui, X. Visible-light-driven photocatalytic disinfection mechanism of Pb-BiFeO3/rGO photocatalyst. Water Res. 2019, 161, 251–261. [Google Scholar] [CrossRef]
- Huiyuan, X.; Fu, W.; Biru, L.; Xiaomin, L.; Jiayu, C.; Yang, Y.; Sen, H.; Xiaoyun, F. A novel double anion layered photocatalyst Pb4(BO3)2SO4 with enhanced photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2022, 429, 132344. [Google Scholar] [CrossRef]
- Saroj, L.; Dushyant, K.P.; Anjali, S.; Shipra, B. Wastewater treatment using nanomaterial quaternary photocatalyst ZrCdPbO4—Removal of colour pollutant by an eco friendly process. J. Indian Chem. Soc. 2021, 98, 100194. [Google Scholar] [CrossRef]
- Jiajia, L.; Ziwei, Z.; Zhuoning, L.; Huijuan, Y.; Shijun, Y.; Yuping, T.; Qizhao, W. Construction of immobilized films photocatalysts with CdS clusters decorated by metal Cd and BiOCl for photocatalytic degradation of tetracycline antibiotics. Chin. Chem. Lett. 2022, 33, 3705–3708. [Google Scholar] [CrossRef]
- Jiajia, L.; Tianyi, L.; Ziwei, Z.; Rui, X.; Yu, L.; Ya, H.; Caiyun, Y.; Sai, Z.; Yuping, T. Preparation of heterostructured ternary Cd/CdS/BiOCl photocatalysts for enhanced visible-light photocatalytic degradation of organic pollutants in wastewater. Inorg. Chem. Commun. 2020, 121, 108236. [Google Scholar] [CrossRef]
- Ya, N.R.; Wei, X.; Lin, X.Z.; Yue, Q.Z. Efficient tetracycline adsorption and photocatalytic degradation of rhodamine B by uranyl coordination polymer. J. Solid State Chem. 2017, 251, 105–112. [Google Scholar] [CrossRef]
- Vinod, K.G.; Shilpi, A.; Deepak, P.; Kothiyal, N.C.; Gaurav, S. Use of pectin–thorium (IV) tungstomolybdate nanocomposite for photocatalytic degradation of methylene blue. Carbohydr. Polym. 2013, 96, 277–283. [Google Scholar] [CrossRef]
- Saikatendu, D.R.; Krishna, C.D.; Siddhartha, S.D. Conventional to green synthesis of magnetic iron oxide nanoparticles; its application as catalyst, photocatalyst and toxicity: A short review. Inorg. Chem. Commun. 2021, 134, 109050. [Google Scholar] [CrossRef]
- Hossein, B. Green synthesis of activated carbon doped tungsten trioxide photocatalysts using leaf of basil (Ocimum basilicum) for photocatalytic degradation of methylene blue under sunlight. J. Saudi Chem. Soc. 2022, 26, 101432. [Google Scholar] [CrossRef]
- Moral-Rodríguez, A.I.; Quintana, M.; Leyva, R.R.; Ojeda-Galvánb, H.J.; Oros, R.S.; Peralta-Rodríguez, R.D.; Mendoza, M.E. Novel and green synthesis of BiVO4 and GO/BiVO4 photocatalysts for efficient dyes degradation under blue LED illumination. Ceram. Int. 2022, 48, 1264–1276. [Google Scholar] [CrossRef]
- Asma, E.G.; Murilo, F.; Nicola, B.; Chérif, D.; Antonio, M.; Michele, O. Wastewater remediation with ZnO photocatalysts: Green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 2021, 286, 112226. [Google Scholar] [CrossRef]
- Lusi, E.; Ruri, A.W.; Hendri, W.; Doty, D.R.; Ade, W.Y.; Rebeka, V.S. Experimental data of CaTiO3 photocatalyst for degradation of organic pollutants (Brilliant green dye)—Green synthesis, characterization and kinetic study. Data Brief 2020, 32, 106099. [Google Scholar] [CrossRef]
- Manjusha, P.; Bonamali, P. A review on CaTiO3 photocatalyst: Activity enhancement methods and photocatalytic applications. Powder Technol. 2021, 3889, 274–304. [Google Scholar] [CrossRef]
- Wenjun, W.; Fawei, L.; Beibei, Y.; Zhanjun, C.; Guanyi, C.; Meng, K.; Chao, Y.; Lian, H. The role of seashell wastes in TiO2/Seashell composites: Photocatalytic degradation of methylene blue dye under sunlight. Environ. Res. 2020, 188, 109831. [Google Scholar] [CrossRef]
- Echabbi, F.; Hamlich, M.; Harkati, S.; Jouali, A.; Tahiri, S.; Lazar, S.; Lakhmiri, R.; Safi, M. Photocatalytic degradation of methylene blue by the use of titanium-doped Calcined Mussel Shells CMS/TiO2. J. Environ. Chem. Eng. 2019, 7, 103293. [Google Scholar] [CrossRef]
- Sanprit, A.; Jaspin, S.; Mahendran, R. Utilization of eggshell waste in calcium-fortified foods and other industrial applications: A review. Trends Food Sci. Technol. 2021, 115, 422–432. [Google Scholar] [CrossRef]
- Jacobs, R.; Gordon, M.; Jerina, M. Feeding a seaweed-derived calcium source versus calcium carbonate on physiological parameters of horses. J. Equine Vet. Sci. 2019, 76, 83. [Google Scholar] [CrossRef]
- Kena, Q.; Qingliang, Z.; Hang, Y.; Xinhui, X.; Jianju, L.; Shufei, H.; Liangliang, W.; Taicheng, A. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Yongjiao, W.; Yiming, H.; Tingting, L.; Jun, C.; Mengfei, L.; Leihong, Z. Photocatalytic degradation of methylene blue on CaBi6O10/Bi2O3 composites under visible light. Chem. Eng. J. 2012, 189–190, 473–481. [Google Scholar] [CrossRef]
- Shtarev, D.S.; Serpone, N. A new generation of visible-light-active photocatalysts—The alkaline earth metal bismuthates: Syntheses, compositions, structures, and properties. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100501. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Kirichenko, E.A.; Kaminsky, O.I.; Makarevich, K.S. Investigation into the efficiency of photocatalytic oxidation of aqueous solutions of organic toxins in a unit with an automatically cleaning bismuth-silicate photocatalyst. J. Water Process Eng. 2020, 37, 101468. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Makarevich, K.S.; Kaminsky, O.I.; Kirichenko, E.A.; Krutikova, V.O. Fabrication of coatings based on strontium-bismuth-silicate photocatalyst for water purification from organic pollutants. Mater. Lett. 2021, 291, 129601. [Google Scholar] [CrossRef]
- Gomez, V.; Rome, B.; Barron, A.R.; Dunnill, C.W. Bi-Phasic photocatalytic particles prepared by sequential layer depositions for water cleaning and purification. Nano Energy Syst. 2016, 5–13. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Astapov, I.A. Prospects for creating regenerated photocatalytic materials for solar water treatment units. Mater. Lett. 2022, 310, 131509. [Google Scholar] [CrossRef]
- Dawei, W.; Miguel, A.M.; José, A.C.M.; Fiderman, M.M.; Ivana, G.; Rodrigo, P.M.M.; Gianluca, L.P. Engineering and modeling perspectives on photocatalytic reactors for water treatment. Water Res. 2021, 202, 117421. [Google Scholar] [CrossRef]
- David, O. Kinetics of photocatalytic, self-cleaning surfaces: A decision tree approach for determination of reaction order. Appl. Catal. B Environ. 2019, 242, 431–440. [Google Scholar] [CrossRef]
- Tannia, V.U.; Heriberto, E.G.; Lucía, Z.F.L.; Gabriel, A.N. Synthesis and characterization of silver nanoparticles supported on Bivalve mollusk shell for catalytic degradation of commercial dyes. J. Photochem. Photobiol. A Chem. 2021, 419, 113481. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Nguyen, T.H.H.; Soda, S.; Vu, N.D.; Bui, T.K.A.; Vo, T.D.H.; Bui, X.T.; Nguyen, T.T.; et al. White hard clam (Meretrix lyrata) shells media to improve phosphorus removal in lab-scale horizontal sub-surface flow constructed wetlands: Performance, removal pathways, and lifespan. Bioresour. Technol. 2020, 312, 123602. [Google Scholar] [CrossRef] [PubMed]
- Imane, H.; Eniko”, T.; Victor, G.M.; Gábor, O.; Gyula, Z.; Said, E.A.; Said, L. Pod razor (Ensis siliqua) shell powder as cost-effective biomineral for removal of nickel (II), copper (II) and zinc (II) from artificially contaminated industrial wastewater. Sustain. Chem. Pharm. 2019, 12, 100137. [Google Scholar] [CrossRef]
- Mohammad, K.O.; Harini, G.; Turki, M.D.; Akshhayya, C.; Asmaa, M.; Abdullah, A.A.; Walid, S.; Mostafa, A.; Hamada, A.E.; Lija, L.R.; et al. Fabrication of MnFe2O4 spheres modified CeO2 nano-flakes for sustainable photodegradation of MB dye and antimicrobial activity: A brief computational investigation on reactive sites and degradation pathway. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128566. [Google Scholar] [CrossRef]
- Haodong, T.; Wujian, Z.; Yue, M.; Bo, X.; Zheming, N.; Shengjie, X. Investigation onto the performance and mechanism of visible light photodegradation of methyl orange catalyzed by M/CeO2 (M = Pt, Ag, Au). Mater. Res. Bull. 2021, 144, 111497. [Google Scholar] [CrossRef]
- Nurfina, Y.; Rahma, A.; Dahlang, T.; Maria, M.S.; Yuliati, H.; Cuk, I.; Munawar, K.; Dede, D. Enhanced photocatalytic degradation of rhodamine 6G (R6G) using ZnO–Ag nanoparticles synthesized by pulsed laser ablation in liquid (PLAL). J. Alloys Compd. 2021, 886, 161291. [Google Scholar] [CrossRef]
- Qingfeng, X.; Ziyao, W.; Hui, Y.; Yajun, X.; Guangjun, N.; Wenjin, Y. Synthesis of hierarchical Cu2CdSnS4 by microwave-assisted transformation from precursor for photodegradation to malachite green. J. Alloys Compd. 2022, 904, 163966. [Google Scholar] [CrossRef]
- Aadil, B.; Deepak, S.; Bonamali, P. Highly efficient CaCO3-CaO extracted from tap water distillation for effective adsorption and photocatalytic degradation of malachite green dye. Mater. Res. Bull. 2019, 116, 1–7. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Amr, M.N.; Tarek, A.S.E.; Ben, A.C. A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: A potent photocatalytic and antibacterial activities. J. Clean. Prod. 2020, 248, 119274. [Google Scholar] [CrossRef]
- Khadija, M.; Muhammad, A.M.; Vickie, M.; Tuan, Z.; Mohd, N.M.Z.; Zarina, A.; Farazila, B.Y. Muhammad Mazhar, Optical and photocatalytic properties of biomimetic cauliflowered Ca2Mn3O8–CaO composite thin films. J. Solid State Chem. 2020, 290, 121552. [Google Scholar] [CrossRef]
- Karen, S.O.G.; Erick, T.A.; Miguel, Á.M.; Fiderman, M.M.; Gianluca, L.P. A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for solar photocatalytic degradation of water contaminants. Chem. Eng. J. 2018, 341, 628–638. [Google Scholar] [CrossRef]
- Lutaenko, K.A. Atlas of Common Bivalve Mollusks of Peter the Great Bay (Sea of Japan); Lutaenko, K.A., Volvenko, I.E., Adrianov, A.V., Eds.; Far Eastern Federal University: Vladivostok, Russia, 2017; p. 140. ISBN 978-5-7444-3980-4. [Google Scholar]
- Plants and Animals of the Japan. In East Sea: Short Field Guide; “Phoenix” Fund, Project Aware (UK), Far Eastern State University: Vladivostok, Russia, 2007; p. 488. ISBN 978-5-74444-1966-0.
- Frédéric, M.; Gilles, L. Molluscan shell proteins. Comptes Rendus Palevol 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Ewelina, K.W.; Jan, M.; Paweł, M. Sintering Behavior of Kaolin with Calcite. Procedia Eng. 2013, 57, 572–582. [Google Scholar] [CrossRef]
- George, T.F. Differentiation of Aragonite from Calcite by Differential Thermal Analysis. Science 1949, 110, 402–403. [Google Scholar] [CrossRef]
- Passe, C.N.; N’Guyen, P.; Pelmard, R.; Ouensanga, A.; Bouchon, C. Water desorption and aragonite—Calcite phase transition in scleractinian corals skeletons. Thermochim. Acta 1995, 265, 135–140. [Google Scholar] [CrossRef]
- Yaozhuang, L.; Yuwei, F.; Zhisheng, X.; Long, Y.; Xiaojiang, X.; Zhengyang, W. Synergistic effect of clam shell bio-filler on the fire-resistance and char formation of intumescent fire-retardant coatings. J. Mater. Res. Technol. 2020, 9, 14718–14728. [Google Scholar] [CrossRef]
- Nur, S.I.; Wai, L.L.; Daud, M.; Siti, H.A.; Hadi, N. A critical review of metal-doped TiO2 and its structure–physical properties–photocatalytic activity relationship in hydrogen production. Int. J. Hydrogen Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Sanakousar, F.M.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Prakashc, K. Recent progress on visible-light-driven metal and non-metal doped ZnO nanostructures for photocatalytic degradation of organic pollutants. Mater. Sci. Semicond. Process. 2022, 140, 106390. [Google Scholar] [CrossRef]
- João, V.N.; Helen, C.F.; Cristiano, P.B. Effect of seawater ionic composition modified by nanofiltration on enhanced oil recovery in Berea sandstone. Fuel 2017, 203, 222–232. [Google Scholar] [CrossRef]
- Jorune, S.; Meaghan, M.; Alberto, J.T.; Matthew, J.C.; Frédéric, M.; Beatrice, D. The degradation of intracrystalline mollusk shell proteins: A proteomics study of Spondylus gaederopus. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2021, 1869, 140718. [Google Scholar] [CrossRef]
- Charles, G. Structure of the conchiolin cases of the prisms in mytilus edulis linne. J. Biophys Biochem. Cytol. 1961, 9, 395–400. [Google Scholar] [CrossRef]
- Hye, W.K.; Taekgeun, Y.; Seungkwan, H.; Seockheon, L.; Seongpil, J. Retardation of wetting for membrane distillation by adjusting major components of seawater. Water Res. 2020, 175, 115677. [Google Scholar] [CrossRef]
- Daniella, M.; Alireza, R.Y.; Marcello, P.; Mark, T. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Xinyue, Z.; Li, L.; Qianlong, Z.; Xingxing, L.; Dongxue, L. Facile synthesis of novel gully-like double-sized mesoporous structural Sr-doped ZrO2–TiO2 composites with improved photocatalytic efficiency. J. Solid State Chem. 2019, 269, 375–385. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Yirui, L.; Ping, Y.; Ting, L. Porous calcite CaCO3 microspheres: Preparation, characterization and release behavior as doxorubicin carrier. Colloids Surf. B Biointerfaces 2020, 186, 110720. [Google Scholar] [CrossRef]
- Eyob, W.; Zelalem, C.L.; Jooheon, K. Polyisocyanate-based water-soluble polyurethane/CaCO3 composites for gunpowder storage. Polym. Test. 2021, 99, 107211. [Google Scholar] [CrossRef]
- Oluwatoosin, B.A.; Agbaje, J.; Gabriel, D.; Dorrit, E.J. Organic biopolymers of venus clams: Collagen-related matrix in the bivalve shells with crossed-lamellar ultrastructure. Biochem. Biophys. Rep. 2021, 26, 100939. [Google Scholar] [CrossRef]
- Emmanuel, J.M.E.; Jonyl, L.G.; Francis, M.d.R.; Eric, R.P. Sonochemical synthesis, characterization and photocatalytic properties of hydroxyapatite nano-rods derived from mussel shells. Mater. Lett. 2017, 196, 33–36. [Google Scholar] [CrossRef]
- Luis, P.V.; Fatah, T.; Laila, M.; Bartolomé, C.H.; Dolores, E.Q.; Pedro, J.S.S. Synthesis of vaterite CaCO3 as submicron and nanosized particles using inorganic precursors and sucrose in aqueous medium. Ceram. Int. 2018, 44, 5291–5296. [Google Scholar] [CrossRef]
- Jun, H.S.; Mark, I.J.; Darrell, A.P. Greener photocatalysts: Hydroxyapatite derived from waste mussel shells for the photocatalytic degradation of a model azo dye wastewater. Chem. Eng. Res. Des. 2013, 91, 1693–1704. [Google Scholar] [CrossRef]
- Ju, Y.L.; Seon, H.J.; Jong, C.L. Effect of surface modification of CaCO3 nanoparticles by a silane coupling agent methyltrimethoxysilane on the stability of foam and emulsion. J. Ind. Eng. Chem. 2019, 74, 63–70. [Google Scholar] [CrossRef]
- Simpson, L.J. Electrochemically generated CaCO3 deposits on iron studied with FTIR and Raman spectroscopy. Electrochim. Acta 1998, 43, 2543–2547. [Google Scholar] [CrossRef]
- Silvia, M.d.P.; Marina, S. Studies on molluscan shells: Contributions from microscopic and analytical methods. Micron. 2009, 40, 669–690. [Google Scholar] [CrossRef]
- Serkan, C. Analysis of grinding aid performance effects on dry fine milling of calcite. Adv. Powder Technol. 2022, 33, 103446. [Google Scholar] [CrossRef]
- Clifford, Y.T.; Meng, C.C.; Shih, W.Y. Synergetic effects of temperature and magnetic field on the aragonite and calcite growth. Chem. Eng. Sci. 2011, 66, 1246–1253. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 16 January 2023).
- Available online: https://www.sciencedirect.com (accessed on 16 January 2023).
- Suguru, N.; Shuntaro, A.; Ryo, S.; Yasuharu, K.; Shinya, Y. Key particle properties of shells for cadmium chemisorption. Chemosphere 2022, 287, 132257. [Google Scholar] [CrossRef]
- Sunil, R.; Virendra, K.S.; Avdesh, S.P.; Mohit, N.; Kuldeep, R. Adsorption of methyl red dye from aqueous solution onto eggshell waste material: Kinetics, isotherms and thermodynamic studies. Curr. Res. Green Sustain. Chem. 2021, 4, 100180. [Google Scholar] [CrossRef]









| Flexural Strength, MPa | Compressive Strength, MPa | Density, g/cm3 | Porosity, % | |||
|---|---|---|---|---|---|---|
| σf(׀׀) | σf(┴) | σf(Average) | σc | ρ | ϕ | |
| Crenomytilus grayanus | 3.38 ± 0.43 | 3.56 ± 0.58 | 3.47 | 0.75 ± 0.23 | 2.65 ± 0.05 | 6.69 |
| Callista brevisiphonata | 1.67 ± 0.35 | 4.34 ± 0.46 | 3.00 | 0.80 ± 0.31 | 2.72 ± 0.01 | 6.88 |
| Mizuhopecten yessoensis | 3.04 ± 0.28 | 4.97 ± 0.86 | 4.00 | 2.36 ± 0.57 | 2.66 ± 0.01 | 2.91 |
| Name of the compound, CAS | Methylene blue CAS: 61-73-4 | Methyl orange CAS: 547-58-0 | Malachite green CAS: 2437-29-8 | Rhodamine 6G CAS: 989-38-8 |
| Class | Acridines | Azo Dyes | Arylmethane Dyes | Xanthenes |
| Chemical structure depiction |  |  |  |  |
| Molecular weight, g/mol | 319.9 | 327.3 | 364.9 | 443.6 |
| The charge of the organic part of the molecule | cation | anion | cation | cation |
| Solubility in water, g/L at 25 °C. | 0.043 | 0.200 | 40 | 1-5 |
| Safety and hazards |  |  |  |  |
| Toxicity LD50(rat), mg/kg | 1180 | 60, oral | 275, oral | debated |
| Number of publications on photocatalytic applications (Since Direct 1998–2022) | >13,000 | >9000 | >1500 | >1100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, A.V.; Astapov, I.A. Study of the Composition and Properties of Bivalve Mollusk Shells as Promising Bio-Indifferent Materials for Photocatalytic Applications (Example of Practical Use). Catalysts 2024, 14, 16. https://doi.org/10.3390/catal14010016
Zaitsev AV, Astapov IA. Study of the Composition and Properties of Bivalve Mollusk Shells as Promising Bio-Indifferent Materials for Photocatalytic Applications (Example of Practical Use). Catalysts. 2024; 14(1):16. https://doi.org/10.3390/catal14010016
Chicago/Turabian StyleZaitsev, Aleksey V., and Ivan A. Astapov. 2024. "Study of the Composition and Properties of Bivalve Mollusk Shells as Promising Bio-Indifferent Materials for Photocatalytic Applications (Example of Practical Use)" Catalysts 14, no. 1: 16. https://doi.org/10.3390/catal14010016