Synergistic Use of Thermostable Laccase and Xylanase in Optimizing the Pre-Bleaching of Kraft Pulp
Abstract
:1. Introduction
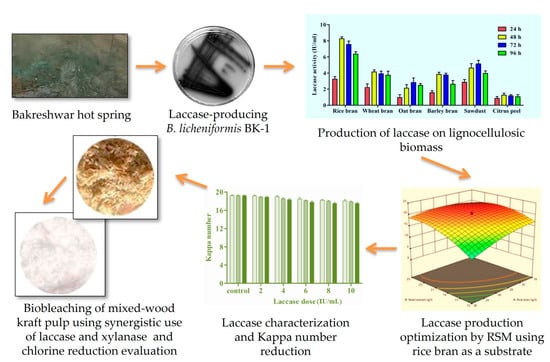
2. Results and Discussion
2.1. Isolation and Identification of Laccase Producer
2.2. Production of Laccase on a Lignocellulosic Substrate via B. licheniformis BK-1
2.3. Optimization of Laccase Production Using Statistical Methods
2.3.1. Screening of Important Components Using the Plackett–Burman (PB) Design
2.3.2. Optimization of Laccase Production via the Central Composite (CC) Design of Response Surface Methodology
AD + 0.7613 BC + 2.02 BD + 1.13 CD − 1.57 A2 − 2.8 B2 − 1.36 C2 − 1.02 D2
2.4. Enzyme Characterization
2.4.1. Effect of pH on Laccase Activity and Stability
2.4.2. Effect of Temperature on Laccase Activity and Stability
2.5. Optimization of Pre-Bleaching Parameters of Laccase
2.6. Influence of Mixture Enzymes (L + X) on the Biobleaching of Mixed-Wood Kraft Pulp
2.6.1. Assessment of Filtrate and Pulp Properties after Enzymatic Treatment
2.6.2. Determination of Optical and Physical Properties after Enzymatic and Chlorine-Based Treatment
2.7. Reduction in Chlorine Use
3. Materials and Methods
3.1. Microbial Strain and Enzyme Production Conditions
3.2. Chemicals and Raw Materials
3.3. Sample Collection and Isolation of a Laccase Producer
3.4. Identification of the Laccase Producer
3.5. Preparation and Evaluation of the Lignocellulosic Substrate for Laccase Production
3.6. Enzyme Assay
3.7. Statistical Optimization of Laccase Production
3.7.1. Plackett–Burman Design
3.7.2. Central Composite Design
3.8. Enzyme Characterization
3.8.1. Effect of pH on Laccase Activity and Stability
3.8.2. Effect of Temperature on Laccase Activity and Stability
3.9. Optimization of Laccase Enzyme Pre-Bleaching Conditions on Kraft Pulp
3.10. Determination of the Biobleaching Effect of Laccase and Xylanase on Kraft Pulp
3.11. Analysis of Filtrate Properties
3.12. Sheet Preparation and Analysis of Optical and Physical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, S.; Yadav, R.D.; Mahajan, R. Synergistic effect of xylano-pectinolytic enzymes produced by a bacterial isolate in bleaching of plywood industrial waste. J. Clean. Prod. 2016, 118, 229–233. [Google Scholar] [CrossRef]
- Parab, P.; Khandeparker, R. Xylanolytic enzyme consortia from Bacillus sp. NIORKP76 for improved biobleaching of kraft pulp. Bioprocess. Biosyst. Eng. 2021, 44, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Patel, N.; Vaghamshi, N.; Shah, K.; Duggirala, S.M.; Dudhagara, P. Trends and strategies in the effluent treatment of pulp and paper industries: A review highlighting reactor options. Curr. Res. Microb. Sci. 2021, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Agrawal, S.; Yadav, R.D.; Mahajan, R. Improved efficacy of ultrafiltered xylanase–pectinase concoction in biobleaching of plywood waste soda pulp. 3 Biotech 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Agrawal, S.; Nagpal, R.; Mishra, O.P.; Bhardwaj, N.; Mahajan, R. Production of eco-friendly and better-quality sugarcane bagasse paper using crude xylanase and pectinase biopulping strategy. 3 Biotech 2023, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Angural, S.; Kumar, A.; Kumar, D.; Warmoota, R.; Sondhi, S.; Gupta, N. Lignolytic and hemicellulolytic enzyme cocktail production from Bacillus tequilensis LXM 55 and its application in pulp biobleaching. Bioprocess. Biosyst. Eng. 2020, 43, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Virk, A.P.; Sharma, P.; Capalash, N. Use of Laccase in pulp and paper industry. Biotechnol. Prog. 2012, 28, 21–32. [Google Scholar] [CrossRef]
- Sridevi, A.; Ramanjaneyulu, G.; Suvarnalatha Devi, P. Biobleaching of paper pulp with xylanase produced by Trichoderma asperellum. 3 Biotech 2017, 7, 266. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, S.; Khatri, M.; Arya, S.K. Biobleaching for pulp and paper industry in India: Emerging enzyme technology. Biocatal. Agric. Biotechnol. 2019, 17, 558–565. [Google Scholar] [CrossRef]
- Boruah, P.; Sarmah, P.; Das, P.K.; Goswami, T. Exploring the lignolytic potential of a new Laccase producing strain Kocuria sp. PBS-1 and its application in bamboo pulp bleaching. Int. Biodeterior. Biodegrad. 2019, 143, 104726. [Google Scholar] [CrossRef]
- Patel, K.; Amaresan, N. Mass Multiplication, Production Cost Analysis and Marketing of Xylanase. In Industrial Microbiology Based Entrepreneurship: Making Money from Microbes; Springer Nature Singapore: Singapore, 2022; pp. 25–35. [Google Scholar]
- Patel, K.; Amaresan, N. Mass Multiplication, Production Cost Analysis, and Marketing of Cellulase. In Industrial Microbiology Based Entrepreneurship: Making Money from Microbes; Springer Nature Singapore: Singapore, 2022; pp. 37–50. [Google Scholar]
- Patel, K.; Dudhagara, P. Optimization of xylanase production by Bacillus tequilensis strain UD-3 using economical agricultural substrate and its application in rice straw pulp bleaching. Biocatal. Agric. Biotechnol. 2020, 30, 101846. [Google Scholar] [CrossRef]
- Pinheiro, V.E.; Ferreira, J.A.; Betini, J.H.A.; Kamimura, E.S.; Polizeli, M.L. Utilizing a novel fungal enzymatic cocktail as an eco-friendly alternative for cellulose pulp biobleaching. BioResources 2021, 16, 7509–7529. [Google Scholar] [CrossRef]
- Desai, M.; Patel, K. Isolation, optimization, and purification of extracellular levansucrase from nonpathogenic Klebsiella strain L1 isolated from waste sugarcane bagasse. Biocatal. Agric. Biotechnol. 2019, 19, 101107. [Google Scholar] [CrossRef]
- Angural, S.; Rana, M.; Sharma, A.; Warmoota, R.; Puri, N.; Gupta, N. Combinatorial biobleaching of mixedwood pulp with lignolytic and hemicellulolytic enzymes for paper making. Indian J. Microbiol. 2022, 60, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Mangrola, A.; Dudhagara, P.; Koringa, P.; Joshi, C.G.; Parmar, M.; Patel, R. Deciphering the microbiota of Tuwa hot spring, India using shotgun metagenomic sequencing approach. Genom. Data 2015, 4, 153–155. [Google Scholar] [CrossRef]
- Bhavsar, S.; Dudhagara, P.; Tank, S. R software package based statistical optimization of process components to simultaneously enhance the bacterial growth, laccase production and textile dye decolorization with cytotoxicity study. PLoS ONE 2018, 13, e0195795. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O. The Sustainable Production of a Novel Laccase from Wheat Bran by Bordetella sp. JWO16: Toward a Total Environment. Catalysts 2021, 11, 677. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Bilal, M.; Chandra, R. Sustainable production of thermostable laccase from agro-residues waste by Bacillus aquimaris AKRC02. Catal. Lett. 2022, 152, 1784–1800. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Carbajo, J.M.; Martín, J.A.; González, A.E.; Villar, J.C. Laccase production by Pycnoporus sanguineus under different culture conditions. J. Basic Microbiol. 2009, 49, 433–440. [Google Scholar] [CrossRef]
- Muthukumarasamy, N.P.; Jackson, B.; Joseph, R.A.; Sevanan, M. Production of extracellular Laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochem. Res. Int. 2015, 2015, 765190. [Google Scholar] [CrossRef]
- Abdelgalil, S.A.; Soliman, N.A.; Abo-Zaid, G.A.; Abdel-Fattah, Y.R. Bioprocessing strategies for cost-effective large-scale production of bacterial Laccase from Lysinibacillus macroides LSO using bio-waste. Int. J. Environ. Sci. Technol. 2021, 19, 1633–1652. [Google Scholar] [CrossRef]
- Prajapati, J.; Dudhagara, P.; Patel, K. Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: Optimization, characterization, and application for fruit juice clarification. Biocatal. Agric. Biotechnol. 2021, 35, 102063. [Google Scholar] [CrossRef]
- Patel, K.; Dudhagara, P. Compatibility testing and enhancing the pulp bleaching process by hydrolases of the newly isolated thermophilic Isoptericola variabilis strain UD-6. Biocatal. Biotransformation 2020, 38, 144–160. [Google Scholar] [CrossRef]
- Patel, H.; Gupte, A.; Gupte, S. Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. BioResources 2009, 4, 268–284. [Google Scholar] [CrossRef]
- Abdullah, R.; Tahseen, M.; Nisar, K.; Kaleem, A.; Iqtedar, M.; Saleem, F.; Aftab, M. Statistical optimization of cellulases by Talaromyces thermophilus utilizing Saccharum spontaneum, a novel substrate. Electron. J. Biotechnol. 2021, 51, 79–87. [Google Scholar] [CrossRef]
- Immerzeel, P.; Fiskari, J. Synergism of enzymes in chemical pulp bleaching from an industrial point of view: A critical review. Can. J. Chem. Eng. 2023, 101, 312–321. [Google Scholar] [CrossRef]
- Mehandia, S.; Sharma, S.C.; Arya, S.K. Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnol. Rep. 2020, 25, e00413. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.; Joseph, B.; Ramteke, P.W. Production of Laccase from newly isolated Pseudomonas putida and its application in bioremediation of synthetic dyes and industrial effluents. Biocatal. Agric. Biotechnol. 2013, 2, 333–338. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Wang, T.N.; Zhao, L.Y.; Du, M.H.; Li, T.L.; Li, D.B. Characterization and dye decolorization ability of an alkaline resistant and organic solvents tolerant Laccase from Bacillus licheniformis LS04. Bioresour. Technol. 2012, 115, 35–40. [Google Scholar] [CrossRef]
- Guan, Z.; Song, C.M.; Zhang, N.; Zhou, W.; Xu, C.W.; Zhou, L.; Zhao, H.; Cia, Y.J.; Liao, X.R. Overexpression, characterization, and dye-decolorizing ability of a thermostable, pH-stable, and organic solvent-tolerant Laccase from Bacillus pumilus W3. J. Mol. Catal. B Enzym. 2014, 101, 1–6. [Google Scholar] [CrossRef]
- Debnath, R.; Mistry, P.; Roy, P.; Roy, B.; Saha, T. Partial purification and characterization of a thermophilic and alkali-stable laccase of Phoma herbarum isolate KU4 with dye-decolorization efficiency. Prep. Biochem. Biotechnol. 2021, 51, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.; Sharma, P.; Saini, S.; Puri, N.; Gupta, N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS ONE 2014, 9, e96951. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, T.; Wang, Q.; Zhang, X.; Peng, H.; Fang, W.; Hong, Y.; Ge, H.; Xiao, Y.A. Bacterial laccase from marine microbial metagenome exhibiting chloride tolerance and dye decolorization ability. Appl. Microbial. Biotechnol. 2011, 89, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Valls, C.; Vidal, T.; Roncero, M.B. Boosting the effect of a laccase–mediator system by using a xylanase stage in pulp bleaching. J. Hazard. Mater. 2010, 177, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Nagpal, R.; Agrawal, S.; Bhardwaj, N.; Mahajan, R. Eco-friendly bleaching of agrowaste wheat straw using crude alkalo-thermotolerant cellulase-free xylano-pectinolytic enzymes. Appl. Biochem. Biotechnol. 2022, 194, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Fillat, U.; Roncero, M.B. Biobleaching of high quality pulps with laccase mediator system: Influence of treatment time and oxygen supply. Biochem. Eng. J. 2022, 44, 193–198. [Google Scholar] [CrossRef]
- Garg, G.; Dhiman, S.S.; Mahajan, R.; Kaur, A.; Sharma, J. Bleach-boosting effect of crude xylanase from Bacillus stearothermophilus SDX on wheat straw pulp. New Biotechnol. 2011, 28, 58–64. [Google Scholar] [CrossRef]
- Nagar, S.; Jain, R.K.; Thakur, V.V.; Gupta, V.K. Biobleaching application of cellulase poor and alkali stable xylanase from Bacillus pumilus SV-85S. 3 Biotech 2013, 3, 277–285. [Google Scholar] [CrossRef]
- Raj, A.; Kumar, S.; Singh, S.K.; Prakash, J. Production and purification of xylanase from alkaliphilic Bacillus licheniformis and its pretreatment of eucalyptus kraft pulp. Biocatal. Agric. Biotechnol. 2018, 15, 199–209. [Google Scholar] [CrossRef]
- Kaur, A.; Varghese, L.M.; Battan, B.; Patra, A.K.; Mandhan, R.P.; Mahajan, R. Bio-degumming of banana fibers using ecofriendly crude xylano-pectinolytic enzymes. Prep. Biochem. Biotechnol. 2020, 50, 521–528. [Google Scholar] [CrossRef]
- Sharma, A.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Enzyme cocktail: An opportunity for greener agro-pulp biobleaching in paper industry. J. Clean. Prod. 2020, 271, 122573. [Google Scholar] [CrossRef]
- Kaur, A.; Mahajan, R.; Singh, A.; Garg, G.; Sharma, J. Application of cellulase-free xylano-pectinolytic enzymes from the same bacterial isolate in biobleaching of kraft pulp. Bioresour. Technol. 2010, 101, 9150–9155. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Q.; Han, S.Y.; Zhang, N.; Hu, H.; Zheng, S.P.; Ye, Y.R.; Lin, Y. Bleach boosting effect of xylanase A from Bacillus halodurans C-125 in ECF bleaching of wheat straw pulp. Enzyme. Microb. Technol. 2013, 52, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N.; Grabski, A.C.; Jeffries, T.W. Chromophore release from kraft pulp by purified Streptomyces roseiscleroticus xylanases. Appl. Microbiol. Biotechnol. 1993, 39, 405–412. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Tappi, A. TAPPI Test Methods, Technical Association of the Pulp and Paper Industry; Tappi Press: Atlanta, GA, USA, 2011. [Google Scholar]
- Babič, J.; Likozar, B.; Pavko, A. Optimization of ligninolytic enzyme activity and production rate with Ceriporiopsis subvermispora for application in bioremediation by varying submerged media composition and growth immobilization support. Inter. J. Mol. Sci. 2012, 13, 11365–11384. [Google Scholar] [CrossRef]
- Hilgers, R.; van Erven, G.; Boerkamp, V.; Sulaeva, I.; Potthast, A.; Kabel, M.A.; Vincken, J.-P. Understanding Laccase/HBT-catalyzed grass delignification at the molecular level. Green Chem. 2020, 22, 1735–1746. [Google Scholar] [CrossRef]
- Cajnko, M.M.; Oblak, J.; Grilc, M.; Likozar, B. Enzymatic bioconversion process of lignin: Mechanisms, reactions and kinetics. Bioresour. Technol. 2021, 340, 125655. [Google Scholar] [CrossRef] [PubMed]







| Run | A: Glucose (g/L) | B: Rice Bran (g/L) | C: Yeast Extract (g/L) | D: K2HPO4 (g/L) | E: MgSO4·7H2O (g/L) | F: NaCl (g/L) | G: CuSO4·5H2O (g/L) | H: CaCl2 (g/L) | I: Temperature (°C) | J: Ph | K: Inoculum Size (%) | Laccase Activity (IU/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | ||||||||||||
| 1 | 0.5 | 5.0 | 15.0 | 0.5 | 0.1 | 1.0 | 0.005 | 0.05 | 30 | 5.0 | 0.5 | 8.32 | 8.47 |
| 2 | 2.0 | 5.0 | 25.0 | 2.0 | 0.4 | 1.0 | 0.005 | 0.05 | 60 | 5.0 | 2.0 | 11.85 | 12.16 |
| 3 | 0.5 | 30.0 | 25.0 | 2.0 | 0.1 | 1.0 | 0.005 | 0.2 | 30 | 10.0 | 2.0 | 12.72 | 12.85 |
| 4 | 2.0 | 5.0 | 25.0 | 2.0 | 0.1 | 4.0 | 0.015 | 0.2 | 30 | 5.0 | 0.5 | 11.79 | 11.67 |
| 5 | 0.5 | 30.0 | 15.0 | 2.0 | 0.4 | 1.0 | 0.015 | 0.2 | 60 | 5.0 | 0.5 | 14.43 | 13.9 |
| 6 | 2.0 | 5.0 | 15.0 | 0.5 | 0.4 | 1.0 | 0.015 | 0.2 | 30 | 10.0 | 2.0 | 8.96 | 9.72 |
| 7 | 2.0 | 30.0 | 15.0 | 2.0 | 0.4 | 4.0 | 0.005 | 0.05 | 30 | 10.0 | 0.5 | 11.39 | 10.9 |
| 8 | 0.5 | 5.0 | 25.0 | 0.5 | 0.4 | 4.0 | 0.005 | 0.2 | 60 | 10.0 | 0.5 | 12.82 | 12.16 |
| 9 | 2.0 | 30.0 | 25.0 | 0.5 | 0.1 | 1.0 | 0.015 | 0.05 | 60 | 10.0 | 0.5 | 15.08 | 15.85 |
| 10 | 2.0 | 30.0 | 15.0 | 0.5 | 0.1 | 4.0 | 0.005 | 0.2 | 60 | 5.0 | 2.0 | 12.1 | 12.65 |
| 11 | 0.5 | 30.0 | 25.0 | 0.5 | 0.4 | 4.0 | 0.015 | 0.05 | 30 | 5.0 | 2.0 | 14.54 | 14.1 |
| 12 | 0.5 | 5.0 | 15.0 | 2.0 | 0.1 | 4.0 | 0.015 | 0.05 | 60 | 10.0 | 2.0 | 11.91 | 11.47 |
| Run | A: Rice Bran | B: Yeast Extract | C: CuSO4·5H2O | D: Temperature | Laccase Activity (IU/mL) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | 0 | 0 | −2 | 0 | 16.54 | 16.86 |
| 2 | 0 | 0 | 0 | 0 | 20.35 | 20.41 |
| 3 | 1 | −1 | −1 | 1 | 16.72 | 16.67 |
| 4 | 1 | −1 | −1 | −1 | 19.46 | 19.41 |
| 5 | 0 | 0 | 0 | 2 | 18.56 | 18.43 |
| 6 | −1 | −1 | 1 | 1 | 6.6 | 6.81 |
| 7 | 0 | 0 | 0 | 0 | 20.68 | 20.41 |
| 8 | 0 | −2 | 0 | 0 | 3.98 | 3.86 |
| 9 | 1 | 1 | 1 | −1 | 9.68 | 9.63 |
| 10 | −1 | 1 | 1 | −1 | 14.22 | 14.32 |
| 11 | −2 | 0 | 0 | 0 | 10.25 | 10.39 |
| 12 | −1 | 1 | −1 | −1 | 13.84 | 13.48 |
| 13 | −1 | −1 | −1 | 1 | 4.6 | 4.48 |
| 14 | 1 | 1 | −1 | 1 | 21.11 | 21.07 |
| 15 | 0 | 0 | 2 | 0 | 13.3 | 13.09 |
| 16 | 0 | 2 | 0 | 0 | 14.36 | 14.59 |
| 17 | 0 | 0 | 0 | 0 | 20.42 | 20.41 |
| 18 | 1 | 1 | −1 | −1 | 15.88 | 15.72 |
| 19 | −1 | −1 | 1 | −1 | 8.12 | 7.99 |
| 20 | −1 | 1 | 1 | 1 | 21.35 | 21.23 |
| 21 | 1 | −1 | 1 | 1 | 11.86 | 12.06 |
| 22 | 1 | 1 | 1 | 1 | 19.45 | 19.5 |
| 23 | 1 | −1 | 1 | −1 | 10.23 | 10.27 |
| 24 | 0 | 0 | 0 | 0 | 20.3 | 20.41 |
| 25 | 2 | 0 | 0 | 0 | 17.91 | 17.88 |
| 26 | −1 | 1 | −1 | 1 | 15.84 | 15.86 |
| 27 | 0 | 0 | 0 | 0 | 20.57 | 20.41 |
| 28 | 0 | 0 | 0 | −2 | 14.02 | 14.26 |
| 29 | −1 | −1 | −1 | −1 | 10.18 | 10.19 |
| 30 | 0 | 0 | 0 | 0 | 20.14 | 20.41 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p > (F) |
|---|---|---|---|---|---|
| Model | 780.7 | 14 | 55.76 | 1041.2 | <0.0001 *** |
| A—Rice bran | 84.23 | 1 | 84.23 | 1572.6 | <0.0001 *** |
| B—Yeast extract | 172.59 | 1 | 172.59 | 3222.54 | <0.0001 *** |
| C—CuSO4·5H2O | 21.28 | 1 | 21.28 | 397.36 | <0.0001 *** |
| D—Temperature | 26.04 | 1 | 26.04 | 486.24 | <0.0001 *** |
| AB | 48.65 | 1 | 48.65 | 908.38 | <0.0001 *** |
| AC | 48.23 | 1 | 48.23 | 900.58 | <0.0001 *** |
| AD | 8.79 | 1 | 8.79 | 164.14 | <0.0001 *** |
| BC | 9.27 | 1 | 9.27 | 173.12 | <0.0001 *** |
| BD | 65.37 | 1 | 65.37 | 1220.5 | <0.0001 *** |
| CD | 20.48 | 1 | 20.48 | 382.31 | <0.0001 *** |
| A2 | 67.52 | 1 | 67.52 | 1260.68 | <0.0001 *** |
| B2 | 214.5 | 1 | 214.5 | 4004.95 | <0.0001 *** |
| C2 | 50.65 | 1 | 50.65 | 945.79 | <0.0001 *** |
| D2 | 28.34 | 1 | 28.34 | 529.13 | <0.0001 *** |
| Residual | 0.8034 | 15 | 0.0536 | ||
| Lack of Fit | 0.6162 | 10 | 0.0616 | 1.65 | 0.3034 ns |
| Pure Error | 0.1872 | 5 | 0.0374 | ||
| Cor Total | 781.51 | 29 |
| Parameters | Control | L + X |
|---|---|---|
| Absorbance at wavelengths (nm) | ||
| 237 | 0.058 ± 0.005 | 1.74 ± 0.01 |
| 280 | 0.064 ± 0.008 | 1.98 ± 0.32 |
| 400 | 0.056 ± 0.004 | 1.45 ± 0.28 |
| 465 | 0.031 ± 0.011 | 1.25 ± 0.07 |
| Reducing sugar (mg/g) | 0.079 ± 0.017 | 2.42 ± 0.19 |
| Kappa number | 19.21 ± 0.12 | 15.87 ± 0.08 |
| Brightness (%) | 22.56 ± 0.23 | 32.43 ± 0.15 |
| Tear index (mN m2/g) | 3.23 ± 0.08 | 4.54 ± 0.36 |
| Burst index (kPa m2/g) | 2.31 ± 0.23 | 3.10 ± 0.41 |
| Tensile strength (kN/m) | 15.8 ± 0.25 | 19.87 ± 0.35 |
| Treatment | Control | Treated L + X | Improvement (%) |
|---|---|---|---|
| Brightness (%) | 77.33 ± 0.52 | 85.31 ± 0.93 *** | 10.39 |
| Tear index (mN m2/g) | 22.78 ± 0.82 | 31.84 ± 0.43 *** | 39.77 |
| Burst index (kPa m2/g) | 12.31 ± 0.44 | 15.12 ± 0.32 *** | 22.82 |
| Tensile strength (kN/m) | 32.77 ± 0.50 | 37.45 ± 0.69 *** | 14.28 |
| Chlorine Dose (%) | Untreated Pulp | L + X Treated Pulp | ||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 100 | 90 | 80 | 70 | 60 | 50 | 40 | |
| Changed in the optical and physical properties | ||||||||
| Brightness (%) | 77.33 | 85.31 | 84.67 | 83.2 | 80.43 | 78.11 | 77.35 | 74.78 |
| Tear index (mN m2/g) | 22.78 | 31.84 | 30.12 | 27.67 | 25.04 | 23.5 | 22.43 | 20.15 |
| Burst index (kPa m2/g) | 12.31 | 15.12 | 14.48 | 14.11 | 13.85 | 13.46 | 12.95 | 10.54 |
| Tensile strength (kN/m) | 32.77 | 37.45 | 36.2 | 35.85 | 35.1 | 34.33 | 33.82 | 30.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, K.; Vaghamshi, N.; Shah, K.; Duggirala, S.M.; Ghelani, A.; Dudhagara, P.; Shyu, D.J.H. Synergistic Use of Thermostable Laccase and Xylanase in Optimizing the Pre-Bleaching of Kraft Pulp. Catalysts 2024, 14, 1. https://doi.org/10.3390/catal14010001
Patel K, Vaghamshi N, Shah K, Duggirala SM, Ghelani A, Dudhagara P, Shyu DJH. Synergistic Use of Thermostable Laccase and Xylanase in Optimizing the Pre-Bleaching of Kraft Pulp. Catalysts. 2024; 14(1):1. https://doi.org/10.3390/catal14010001
Chicago/Turabian StylePatel, Kartik, Nilam Vaghamshi, Kamlesh Shah, Srinivas Murty Duggirala, Anjana Ghelani, Pravin Dudhagara, and Douglas J. H. Shyu. 2024. "Synergistic Use of Thermostable Laccase and Xylanase in Optimizing the Pre-Bleaching of Kraft Pulp" Catalysts 14, no. 1: 1. https://doi.org/10.3390/catal14010001