Microwave-Assisted Catalytic Conversion of 5-HMF for Biofuel Additives by Molybdophosphoric Acid Encapsulated KCC-1
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization Analysis
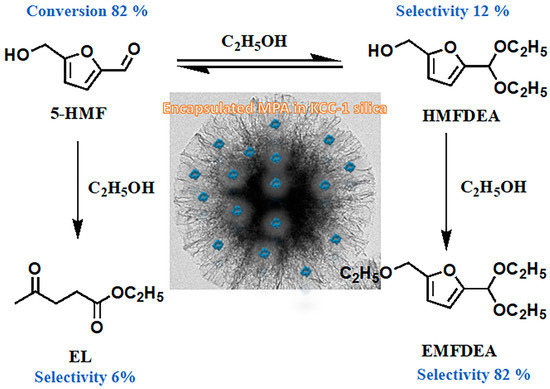
2.2. Catalytic Activity Study on HMF Etherification and Acylation with Ethanol
3. Experimental
3.1. Chemicals
3.2. Synthesis Procedure of MPA Encapsulated KCC-1
3.3. Characterizations
3.4. Activity and Product Analysis of Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.L.; Varbanov, P.; Klemeš, J. Minimising carbon footprint of regional biomass supply chains. Resour. Conserv. Recycl. 2010, 54, 303–309. [Google Scholar] [CrossRef]
- Nie, Y.; Li, J.; Wang, C.; Huang, G.; Fu, J.; Chang, S.; Li, H.; Ma, S.; Yu, L.; Cui, X.; et al. A fine-resolution estimation of the biomass resource potential across China from 2020 to 2100. Resour. Conserv. Recycl. 2022, 176, 105944. [Google Scholar] [CrossRef]
- Opia, A.C.; Hamid, M.K.B.A.; Syahrullail, S.; Rahim, A.B.A.; Johnson, C.A.N. Biomass as a potential source of sustainable fuel, chemical and tribological materials—Overview. Mater. Today Proc. 2021, 39, 922–928. [Google Scholar] [CrossRef]
- Mittal, A.; Pilath, H.M.; Johnson, D.K. Direct Conversion of Biomass Carbohydrates to Platform Chemicals: 5-Hydroxymethylfurfural (HMF) and Furfural. Energy Fuels 2020, 34, 3284–3293. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic Conversion of Renewable Biomass Resources to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butler, I.S.; Xu, L.; Song, H.; Wei, X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chem. 2018, 20, 3657–3682. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sustain. Energy Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Alipour, S.; Omidvarborna, H.; Kim, D.-S. A review on synthesis of alkoxymethyl furfural, a biofuel candidate. Renew. Sustain. Energy Rev. 2017, 71, 908–926. [Google Scholar] [CrossRef]
- Fan, W.; Verrier, C.; Queneau, Y.; Popowycz, F. 5-Hydroxymethylfurfural (HMF) in Organic Synthesis: A Review of its Recent Applications Towards Fine Chemicals. Curr. Org. Synth. 2019, 16, 583–614. [Google Scholar] [CrossRef]
- Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Biomass-Derived Chemicals: Synthesis of Biodegradable Surfactant Ether Molecules from Hydroxymethylfurfural. ChemSusChem 2014, 7, 210–220. [Google Scholar] [CrossRef]
- Sacia, E.R.; Balakrishnan, M.; Bell, A.T. Biomass conversion to diesel via the etherification of furanyl alcohols catalyzed by Amberlyst-15. J. Catal. 2014, 313, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, L.; Wang, S.; Wu, Y. Recent Advances in Aqueous-Phase Catalytic Conversions of Biomass Platform Chemicals Over Heterogeneous Catalysts. Front. Chem. 2020, 7, 948. [Google Scholar] [CrossRef] [Green Version]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G.J.C.T. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Allen, M.C.; Hoffman, A.J.; Liu, T.-w.; Webber, M.S.; Hibbitts, D.; Schwartz, T.J. Highly Selective Cross-Etherification of 5-Hydroxymethylfurfural with Ethanol. ACS Catal. 2020, 10, 6771–6785. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Nefedov, O.M.; Kustov, L.M. Metal–Organic Frameworks-Based Catalysts for Biomass Processing. Catalysts 2018, 8, 368. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Wang, X.; Huang, C.; Li, Y.; Chen, B. Facile, One-Pot, Two-Step, Strategy for the Production of Potential Bio-Diesel Candidates from Fructose. Catalysts 2017, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- Ayashi, N.; Najafi Chermahini, A.; Saraji, M. Biomass conversion to alkyl levulinates using heteropoly acid carbon mesoporous composites. Process Saf. Environ. Prot. 2022, 160, 988–1000. [Google Scholar] [CrossRef]
- Afshari, M.; Varma, R.S.; Saghanezhad, S.J. Catalytic Applications of Heteropoly acid-Supported Nanomaterials in Synthetic Transformations and Environmental Remediation. Comments Inorg. Chem. 2022, 43, 129–176. [Google Scholar] [CrossRef]
- Boahene, P.E.; Vedachalam, S.; Dalai, A.K. Catalytic oxidative desulfurization of light gas oil over Keggin-type phosphomolybdic acid supported on TUD-1 metallosilicates. Fuel 2022, 317, 123447. [Google Scholar] [CrossRef]
- Chhabra, T.; Rohilla, J.; Krishnan, V. Nanoarchitectonics of phosphomolybdic acid supported on activated charcoal for selective conversion of furfuryl alcohol and levulinic acid to alkyl levulinates. Mol. Catal. 2022, 519, 112135. [Google Scholar] [CrossRef]
- Zhou, S.; He, J.; Wu, P.; He, L.; Tao, D.; Lu, L.; Yu, Z.; Zhu, L.; Chao, Y.; Zhu, W. Metal-organic framework encapsulated high-loaded phosphomolybdic acid: A highly stable catalyst for oxidative desulfurization of 4,6-dimethyldibenzothiophene. Fuel 2022, 309, 122143. [Google Scholar] [CrossRef]
- Winoto, H.P.; Fikri, Z.A.; Ha, J.-M.; Park, Y.-K.; Lee, H.; Suh, D.J.; Jae, J. Heteropolyacid supported on Zr-Beta zeolite as an active catalyst for one-pot transformation of furfural to γ-valerolactone. Appl. Catal. B Environ. 2019, 241, 588–597. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. Esterification of lauric acid with butanol-1 over H3PW12O40 supported on MCM-41. Fuel 2012, 102, 72–77. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Bakkali, B.E.; Trautwein, G.; Reinoso, S. Zirconia-supported tungstophosphoric heteropolyacid as heterogeneous acid catalyst for biodiesel production. Appl. Catal. B Environ. 2018, 224, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.P.d.; Gomes Aciole, R.C.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Andrade Pacheco, J.G.; Lopes Barros, I.d.C. Residue-based activated carbon from passion fruit seed as support to H3PW12O40 for the esterification of oleic acid. J. Clean. Prod. 2021, 282, 124477. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [Green Version]
- Cerón-Camacho, R.; Aburto, J.A.; Montiel, L.E.; Martínez-Palou, R. Microwave-assisted organic synthesis versus conventional heating. A comparative study for Fisher glycosidation of monosaccharides. Comptes Rendus Chim. 2013, 16, 427–432. [Google Scholar] [CrossRef]
- Remón, J.; Randall, J.; Budarin, V.L.; Clark, J.H. Production of bio-fuels and chemicals by microwave-assisted, catalytic, hydrothermal liquefaction (MAC-HTL) of a mixture of pine and spruce biomass. Green Chem. 2019, 21, 284–299. [Google Scholar] [CrossRef]
- Vasudevan, S.V.; Kong, X.; Cao, M.; Wang, M.; Mao, H.; Bu, Q. Microwave-assisted liquefaction of carbohydrates for 5-hydroxymethylfurfural using tungstophosphoric acid encapsulated dendritic fibrous mesoporous silica as a catalyst. Sci. Total Environ. 2021, 760, 143379. [Google Scholar] [CrossRef]
- Sudhakar, P.; Pandurangan, A. Heteropolyacid (H3PW12O40)-impregnated mesoporous KIT-6 catalyst for green synthesis of bio-diesel using transesterification of non-edible neem oil. Mater. Renew. Sustain. Energy 2019, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, S.; Ikeda, T.; Koutani, M.; Yasumura, S.; Amakawa, K.; Shimoda, K.; Jing, Y.; Toyao, T.; Sadakane, M.; Shimizu, K.-I.; et al. Oxidation Catalysis over Solid-State Keggin-Type Phosphomolybdic Acid with Oxygen Defects. J. Am. Chem. Soc. 2022, 144, 7693–7708. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; da Silva, M.J.; Rodrigues, A.A.; Ferreira, S.O.; da Silva, R.C. Vanadium-doped sodium phosphomolybdate salts as catalysts in the terpene alcohols oxidation with hydrogen peroxide. RSC Adv. 2021, 11, 24072–24085. [Google Scholar] [CrossRef]
- Huang, X.; Tao, Z.; Praskavich, J.C.; Goswami, A.; Al-Sharab, J.F.; Minko, T.; Polshettiwar, V.; Asefa, T. Dendritic Silica Nanomaterials (KCC-1) with Fibrous Pore Structure Possess High DNA Adsorption Capacity and Effectively Deliver Genes In Vitro. Langmuir 2014, 30, 10886–10898. [Google Scholar] [CrossRef]
- Tonutti, L.G.; Dalla Costa, B.O.; Mendow, G.; Pestana, G.L.; Veizaga, N.S.; Grau, J.M. Etherification of hydroxymethylfurfural with ethanol on mesoporous silica catalysts of regulated acidity to obtain ethoxymethylfurfural, a bio-additive for diesel. Microporous Mesoporous Mater. 2022, 343, 112145. [Google Scholar] [CrossRef]
- Guo, H.; Dowaki, T.; Shen, F.; Qi, X.; Smith, R.L. Critical Assessment of Reaction Pathways for Next-Generation Biofuels from Renewable Resources: 5-Ethoxymethylfurfural. ACS Sustain. Chem. Eng. 2022, 10, 9002–9021. [Google Scholar] [CrossRef]











| Catalyst | SBET a (m²/g) | Vtp b (cm3/g) | dP, BJH c (nm) | Total Acidity d mmol NH3/g |
|---|---|---|---|---|
| KCC-1 | 523 | 1.63 | 13 | 0.022 |
| MPA-KCC-1 | 320 | 0.65 | 14 | 0.251 |
| IMPA-KCC-1 | 380 | 1.20 | 13 | 0.385 |
| No. of Cycles | 5-HMF Conversion (%) a | Selectivity b(%) | ||
|---|---|---|---|---|
| HMFDEA | EMFDEA | EL | ||
| 1 | 82 | 13 | 82 | 5 |
| 2 | 80 | 12 | 84 | 4 |
| 3 | 79 | 10 | 83 | 7 |
| 4 | 73 | 14 | 81 | 3 |
| 5 | 75 | 12 | 82 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasudevan, S.V.; Cai, J.; Xu, J.; Lin, H.; Wang, H.; Bu, Q. Microwave-Assisted Catalytic Conversion of 5-HMF for Biofuel Additives by Molybdophosphoric Acid Encapsulated KCC-1. Catalysts 2023, 13, 969. https://doi.org/10.3390/catal13060969
Vasudevan SV, Cai J, Xu J, Lin H, Wang H, Bu Q. Microwave-Assisted Catalytic Conversion of 5-HMF for Biofuel Additives by Molybdophosphoric Acid Encapsulated KCC-1. Catalysts. 2023; 13(6):969. https://doi.org/10.3390/catal13060969
Chicago/Turabian StyleVasudevan, Srinivasan Vinju, Jin Cai, Junming Xu, Hongjian Lin, Hongliang Wang, and Quan Bu. 2023. "Microwave-Assisted Catalytic Conversion of 5-HMF for Biofuel Additives by Molybdophosphoric Acid Encapsulated KCC-1" Catalysts 13, no. 6: 969. https://doi.org/10.3390/catal13060969