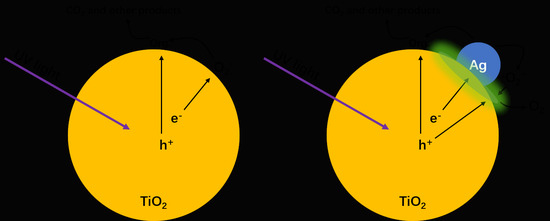
Atmosphere-Dependent Electron Relaxation of the Ag-Decorated TiO2 and the Relations with Photocatalytic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Characterizations
2.2. Atmosphere-Dependent Photoconductances and Relaxation Kinetics
2.2.1. Photoconductances in Pure N2 Atmosphere
2.2.2. Photoconductances in the Methanol-Containing N2 Atmosphere
2.3. Atmosphere-Dependent Photoinduced Absorption and Relaxation Kinetics
2.3.1. Diffusion Absorption in N2 Atmosphere
2.3.2. Photoinduced Absorption in the Methanol-Containing N2 Atmosphere
2.4. Relaxation Pathways
2.5. Photocatalytic Properties and the Relation with the Electron Relaxation
3. Experimental Section
3.1. Sample Preparation
3.2. Catalyst Characterization
3.3. In Situ Photoconductance Measurement
3.4. In Situ Diffuse Reflection Measurement
3.5. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sust. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Combination effect of activated carbon with TiO2 for the photodegradation of binary pollutants at typical indoor air level. J. Photochem. Photobiol. A 2004, 161, 131–140. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Yoshida, H. Semiconductor Photocatalysts for Non-oxidative Coupling, Dry Reforming and Steam Reforming of Methane. Catal. Surv. Asia 2014, 18, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Walenta, C.A.; Courtois, C.; Kollmannsberger, S.L.; Eder, M.; Tschurl, M.; Heiz, U. Surface Species in Photocatalytic Methanol Reforming on Pt/TiO2(110): Learning from Surface Science Experiments for Catalytically Relevant Conditions. ACS Catal. 2020, 10, 4080–4091. [Google Scholar] [CrossRef]
- Wang, X.; Rosspeintner, A.; Ziarati, A.; Zhao, J.; Bürgi, T. Insight into the transient inactivation effect on Au/TiO2 catalyst by in-situ DRIFT and UV–vis spectroscopy. Nat. Commun. 2022, 13, 5458. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef]
- Liu, H.; Sun, C.; Fan, Z.; Jia, X.; Sun, J.; Gao, F.; Tang, C.; Dong, L. Doping effect of Sm on the TiO2/CeSmOx catalyst in the NH3-SCR reaction: Structure–activity relationship, reaction mechanism and SO2 tolerance. Catal. Sci. Technol. 2019, 9, 3554–3567. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, Y.; Wang, H.; Zhang, C.; Li, G.; Zou, L. A photoelectrochemical sensor for glutathione based on Bi2S3-modified TiO2 nanotube arrays. New J. Chem. 2022, 46, 8162–8170. [Google Scholar] [CrossRef]
- Fu, S.; Qiao, D.; Luo, Y.; Jiao, Z.; Cheng, L. Heteroatom Modified TiO2/Graphene Aerogel Composites by Electron Beam Irradiation in Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 6312–6322. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.k.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bian, Z.; Zhu, J.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Au/TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 4538–4539. [Google Scholar] [CrossRef] [PubMed]
- Li, F.B.; Li, X.Z. The enhancement of photodegradation efficiency using Pt–TiO2 catalyst. Chemosphere 2002, 48, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Li, Y.; Wang, Y.; He, H. Sodium-Promoted Pd/TiO2 for Catalytic Oxidation of Formaldehyde at Ambient Temperature. Environ. Sci. Technol. 2014, 48, 5816–5822. [Google Scholar] [CrossRef]
- Nelson, R.C.; Baek, B.; Ruiz, P.; Goundie, B.; Brooks, A.; Wheeler, M.C.; Frederick, B.G.; Grabow, L.C.; Austin, R.N. Experimental and Theoretical Insights into the Hydrogen-Efficient Direct Hydrodeoxygenation Mechanism of Phenol over Ru/TiO2. ACS Catal. 2015, 5, 6509–6523. [Google Scholar] [CrossRef]
- Takechi, K.; Sudeep, P.K.; Kamat, P.V. Harvesting Infrared Photons with Tricarbocyanine Dye Clusters. J. Phys. Chem. B 2006, 110, 16169–16173. [Google Scholar] [CrossRef]
- Dai, X.; Jiao, Z.; Ma, Z.; Liu, K.; Wang, C.; Su, H. Capturing the Long-Lived Photogenerated Electrons in Au/TiO2 upon UV or Visible Irradiation by Time-Resolved Infrared Spectroscopy. J. Phys. Chem. C 2019, 123, 20325–20332. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.-A.; Onishi, H. Water- and Oxygen-Induced Decay Kinetics of Photogenerated Electrons in TiO2 and Pt/TiO2: A Time-Resolved Infrared Absorption Study. J. Phys. Chem. B 2001, 105, 7258–7262. [Google Scholar] [CrossRef]
- Chuang, H.-Y.; Chen, D.-H. Fabrication and photocatalytic activities in visible and UV light regions of Ag@TiO2 and NiAg@TiO2 nanoparticles. Nanotechnology 2009, 20, 105704. [Google Scholar] [CrossRef]
- Liu, B.; Wu, H.; Zhang, X.; Parkin, I.P.; Zhao, X. New Insight into the Role of Electron Transfer to O2 in Photocatalytic Oxidations of Acetone over TiO2 and the Effect of Au Cocatalyst. J. Phys. Chem. C 2019, 123, 30958–30971. [Google Scholar] [CrossRef]
- van Turnhout, L.; Hattori, Y.; Meng, J.; Zheng, K.; Sá, J. Direct Observation of a Plasmon-Induced Hot Electron Flow in a Multimetallic Nanostructure. Nano Lett. 2020, 20, 8220–8228. [Google Scholar] [CrossRef]
- Song, J.; Long, J.; Liu, Y.; Xu, Z.; Ge, A.; Piercy, B.D.; Cullen, D.A.; Ivanov, I.N.; McBride, J.R.; Losego, M.D.; et al. Highly Efficient Plasmon Induced Hot-Electron Transfer at Ag/TiO2 Interface. ACS Photonics 2021, 8, 1497–1504. [Google Scholar] [CrossRef]
- Yoshihara, T.; Katoh, R.; Furube, A.; Tamaki, Y.; Murai, M.; Hara, K.; Murata, S.; Arakawa, H.; Tachiya, M. Identification of Reactive Species in Photoexcited Nanocrystalline TiO2 Films by Wide-Wavelength-Range (400–2500 nm) Transient Absorption Spectroscopy. J. Phys. Chem. B 2004, 108, 3817–3823. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Parkin, I.P.; Zhao, X. The effect of Cu dopants on electron transfer to O2 and the connection with acetone photocatalytic oxidations over nano-TiO2. Phys. Chem. Chem. Phys. 2021, 23, 8300–8308. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Miao, S.; Liu, T.; Mu, L.; Li, R.; Fan, F.; Li, C. Positioning the Water Oxidation Reaction Sites in Plasmonic Photocatalysts. J. Am. Chem. Soc. 2017, 139, 11771–11778. [Google Scholar] [CrossRef]
- Zhao, B.; Wen, L.; Xu, L.; Zhao, X.; Liu, B. The Effect of Cu(II) Nanoparticle Decoration on the Electron Relaxations and Gaseous Photocatalytic Oxidations of Nanocrystalline TiO2. Catalysts 2023, 13, 550. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Chen, D.; Lv, X.; Li, J. Tuning Photoelectrochemical Performances of Ag-TiO2 Nanocomposites via Reduction/Oxidation of Ag. Chem. Mater. 2008, 20, 6543–6549. [Google Scholar] [CrossRef]
- Zhang, F.; Guan, N.; Li, Y.; Zhang, X.; Chen, J.; Zeng, H. Control of Morphology of Silver Clusters Coated on Titanium Dioxide during Photocatalysis. Langmuir 2003, 19, 8230–8234. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Zhou, X.; Parkin, I.P.; Zhao, X.; Liu, B. A light–heat synergism in the sub-bandgap photocatalytic response of pristine TiO2: A study of in situ diffusion reflectance and conductance. Phys. Chem. Chem. Phys. 2022, 24, 5618–5626. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, L.; Wu, Z.; Zhou, X.; Zhao, X.; Liu, B. Plasmon-assisted facile selective gaseous isopropanol dehydrogenation over Ag nanocubes. Catal. Sci. Technol. 2022, 12, 94–104. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Wen, L.; Liu, B. Atmosphere-Dependent Electron Relaxation of the Ag-Decorated TiO2 and the Relations with Photocatalytic Properties. Catalysts 2023, 13, 970. https://doi.org/10.3390/catal13060970
Zhao W, Wen L, Liu B. Atmosphere-Dependent Electron Relaxation of the Ag-Decorated TiO2 and the Relations with Photocatalytic Properties. Catalysts. 2023; 13(6):970. https://doi.org/10.3390/catal13060970
Chicago/Turabian StyleZhao, Wenhao, Liping Wen, and Baoshun Liu. 2023. "Atmosphere-Dependent Electron Relaxation of the Ag-Decorated TiO2 and the Relations with Photocatalytic Properties" Catalysts 13, no. 6: 970. https://doi.org/10.3390/catal13060970