Ruthenium Metathesis Catalysts with Imidazole Ligands
Abstract
:1. Introduction
2. Results and Discussion
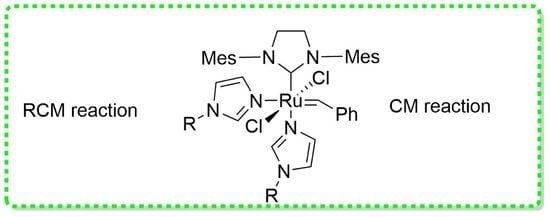
2.1. Synthesis of Catalysts 6a and 6b
2.2. RCM Transformations
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Catalytic Tests
3.2.1. RCM Reactions
3.2.2. CM Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoveyda, A.H.; Zhugralin, A.R. The Remarkable Metal-catalysed Olefin Metathesis Reaction. Nature 2007, 450, 243–251. [Google Scholar] [CrossRef]
- Olefin Metathesis: Theory and Practice; Grela, K. (Ed.) Wiley & Sons, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Handbook of Metathesis; Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. (Eds.) Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Higman, C.S.; Lummiss, J.A.M.; Fogg, D.E. Olefin Metathesis at the Dawn of Implementation in Pharmaceutical and SpecialtyChemicals Manufacturing. Angew. Chem. Int. Ed. 2016, 55, 3552–3565. [Google Scholar] [CrossRef]
- Turczel, G.; Kovács, E.; Merza, G.; Coish, P.; Anastas, P.T.; Tuba, R. Synthesis of Semiochemicals via Olefin Metathesis. ACS Sustain. Chem. Eng. 2019, 7, 33–48. [Google Scholar] [CrossRef]
- Ahmed, W.; Jayant, V.; Alvi, S.; Ahmed, N.; Ahmed, A.; Ali, R. Metathesis Reactions in Natural Product Fragments and Total Syntheses. Asian J. Org. Chem. 2022, 11, e202100753. [Google Scholar] [CrossRef]
- Huang, J.; Stevens, E.D.; Nolan, S.P.; Petersen, J.L. Olefin Metathesis-Active Ruthenium Complexes Bearing a Nucleophilic Carbene Ligand. J. Am. Chem. Soc. 1999, 121, 2674–2678. [Google Scholar] [CrossRef]
- Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H. Increased Ring Closing Metathesis Activity of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with Imidazolin-2-ylidene Ligands. Tetrahedron Lett. 1999, 40, 2247–2250. [Google Scholar] [CrossRef]
- Ackermann, L.; Fürstner, A.; Weskamp, T.; Kohl, F.J.; Herrmann, W.A. Ruthenium Carbene Complexes with Imidazolin-2-ylidene Ligands Allow the Formation of Tetrasubstituted Cycloalkenes by RCM. Tetrahedron Lett. 1999, 40, 4787–4790. [Google Scholar] [CrossRef]
- Love, J.A.; Morgan, J.P.; Trnka, T.M.; Grubbs, R.H. A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile. Angew. Chem. Int. Ed. 2002, 41, 4035–4037. [Google Scholar] [CrossRef]
- Garber, B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. [Google Scholar] [CrossRef]
- Sanford, M.S.; Love, J.A.; Grubbs, R.H. A Versatile Precursor for the Synthesis of New Ruthenium Olefin Metathesis Catalysts. Organometallics 2001, 20, 5314–5318. [Google Scholar] [CrossRef] [Green Version]
- Żukowska, K.; Szadkowska, A.; Pazio, A.E.; Woźnia, K.; Grela, K. Thermal Switchability of N-Chelating Hoveyda-type Catalyst Containing a Secondary Amine Ligand. Organometallics 2012, 31, 462–469. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Tzur, E.; Ben-Asuly, A.; Goldberg, I.; Straub, B.F.; Lemcoffff, N.G. Predicting the Cis—Trans Dichloro Configuration of Group 15–16 Chelated Ruthenium Olefin Metathesis Complexes: A DFT and Experimental Study. Inorg. Chem. 2009, 48, 10819–10825. [Google Scholar] [CrossRef]
- Tzur, E.; Szadkowska, A.; Ben-Asuly, A.; Makal, A.; Goldberg, I.; Woźniak, K.; Grela, K.; Lemcoffff, N.G. Studies on Electronic Effects in O-, N- and S-Chelated Ruthenium Olefin-Metathesis Catalysts. Chem.-Eur. J. 2010, 16, 8726–8737. [Google Scholar] [CrossRef]
- Barbasiewicz, M.; Szadkowska, A.; Bujok, R.; Grela, K. Structure and Activity Peculiarities of Ruthenium Quinoline and Quinoxaline Complexes: Novel Metathesis Catalysts. Organometallics 2006, 25, 3599–3604. [Google Scholar] [CrossRef]
- Williams, M.J.; Kong, J.; Chung, C.K.; Brunskill, A.; Campeau, L.C.; McLaughlin, M. The Discovery of Quinoxaline-Based Metathesis Catalysts from Synthesis of Grazoprevir (MK-5172). Org. Lett. 2016, 18, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.; Hejl, A.; Grubbs, R.H.; Schrodi, Y. Latent Ruthenium Olefin Metathesis Catalysts That Contain an N-Heterocyclic Carbene Ligand. Organometallics 2004, 23, 5399–5401. [Google Scholar] [CrossRef] [Green Version]
- Slugovc, C.; Burtscher, D.; Stelzer, F.; Mereiter, K. Thermally Switchable Olefin Metathesis Initiators Bearing Chelating Carbenes: Influence of the Chelate’s Ring Size. Organometallics 2005, 24, 2255–2258. [Google Scholar] [CrossRef]
- Hejl, A.; Day, M.W.; Grubbs, R.H. Latent Olefin Metathesis Catalysts Featuring Chelating Alkylidenes. Organometallics 2006, 25, 6149–6154. [Google Scholar] [CrossRef] [Green Version]
- Jordaan, M.; Vosloo, H.C.M. Ruthenium Catalyst with a Chelating Pyridinyl-Alcoholato Ligand for Application in Linear Alkene Metathesis. Adv. Synth. Catal. 2007, 349, 184–192. [Google Scholar] [CrossRef]
- Szadkowska, A.; Gstrein, X.; Burtscher, D.; Jarzembska, K.; Wozniak, K.; Slugovc, C.; Grela, K. Latent Thermo-Switchable Olefin Metathesis Initiators Bearing a Pyridyl-Functionalized Chelating Carbene: Influence of the Leaving Group’s Rigidity on the Catalyst’s Performance. Organometallics 2010, 29, 117–124. [Google Scholar] [CrossRef]
- Pump, E.; Leitgeb, A.; KozÉ«owska, A.; Torvisco, A.; Falivene, L.; Cavallo, L.; Grela, K.; Slugovc, C. Variation of the Sterical Properties of the N-Heterocyclic Carbene Coligand in Thermally Triggerable Ruthenium-Based Olefin Metathesis Precatalysts/Initiators. Organometallics 2015, 34, 5383–5392. [Google Scholar] [CrossRef]
- Duan, Y.-L.; Wang, T.; Xie, Q.-X.; Yu, X.-B.; Guo, W.-J.; Wang, J.-H.; Liu, G.-Y. Highly Efficient Nitrogen Chelated Ruthenium Carbene Metathesis Catalysts. Dalton Trans. 2016, 45, 19441. [Google Scholar] [CrossRef]
- Wappel, J.; Fischer, R.C.; Cavallo, L.; Slugovc, C.; Poater, A. Poater Simple Activation by Acid of Latent Ru-NHC-Based Metathesis Initiators Bearing 8-Quinolinolate Co-Ligands. Beilstein J. Org. Chem. 2016, 12, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Czarnocki, S.J.; Czeluśniak, I.; Olszewski, T.K.; Malinska, M.; Woźniak, K.; Grela, K. Rational and Then Serendipitous Formation of Aza Analogues of Hoveyda-Type Catalysts Containing a Chelating Ester Group Leading to a Polymerization Catalyst Family. ACS Catal. 2017, 7, 4115–4121. [Google Scholar] [CrossRef]
- Szwaczko, K.; Czeluśniak, I.; Grela, K. A partially serendipitous Discovery of Thermo-Switchable Ruthenium Olefin Metathesis Initiator That Seem to Be Well Suited for ROMP of Monomers Bearing Vinyl Pendant Groups. J. Organomet. Chem. 2017, 847, 146–153. [Google Scholar] [CrossRef]
- Tole, T.T.; Du Toit, J.I.; Sittert, C.V.; Jordaan, J.; Vosloo, H. Synthesis and Application of Novel Ruthenium Catalysts for High Temperature Alkene Metathesis. Catalysts 2017, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Higman, C.S.; Nascimento, D.L.; Ireland, B.J.; Audorsch, S.; Bailey, G.A.; McDonald, R.; Fogg, D.E. Chelate-Assisted Ring-Closing Metathesis: A Strategy for Accelerating Macrocyclization at Ambient Temperatures. J. Am. Chem. Soc. 2018, 140, 1604–1607. [Google Scholar] [CrossRef]
- Rogalski, S.; Żak, P.; Pawluć, P.; Kubicki, M. Carbamato-Benzylidene Ruthenium Chelates—Synthesis, Structure and Catalytic Activity in Olefin. J. Organomet. Chem. 2018, 859, 1–9. [Google Scholar] [CrossRef]
- Gawin, A.; Pump, E.; Slugovc, C.; Kajetanowicz, A.; Grela, K. Ruthenium Amide Complexes—Synthesis and Catalytic Activity in Olefin Metathesis and in Ring-Opening Polymerisation. Eur. J. Inorg. Chem. 2018, 16, 1766–1774. [Google Scholar] [CrossRef]
- Joo, W.; Chen, C.H.; Moerdyk, J.P.; Deschner, R.P.; Bielawski, C.W.; Willson, C.G. Photoinitiated Ring-Opening Metathesis Polymerization. J. Polym. Sci. Pol. Chem. 2019, 57, 1791–1795. [Google Scholar] [CrossRef] [Green Version]
- Smit, W.; Ekeli, J.B.; Occhipinti, G.; Woźniak, B.; Törnroos, K.W.; Jensen, V.R. Z-Selective Monothiolate Ruthenium Indenylidene Olefin Metathesis Catalysts. Organometallics 2020, 39, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Cena, N.; Schrodi, Y. Robust Olefin Metathesis Catalyst Bearing a Tridentate Hemilabile NHC Ligand. Organometallics 2020, 39, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, D.; Seo, B.; Lim, C.-S.; Kim, H.; Kim, H.-G. Intramolecular Hydrogen-Bond-Based Latent Initiator for Olefin Metathesis Polymerization. Organometallics 2021, 40, 314–323. [Google Scholar] [CrossRef]
- Kumandin, P.A.; Antonova, A.S.; Alekseeva, K.A.; Nikitina, E.V.; Novikov, R.A.; Vasilyev, K.A.; Sinelshchikova, A.A.; Grigoriev, M.S.; Polyanskii, K.B.; Zubkov, F.I. Influence of the N→Ru Coordinate Bond Length on the Activity of New Types of Hoveyda–Grubbs Olefin Metathesis Catalysts Containing a Six-Membered Chelate Ring Possessing a Ruthenium–Nitrogen Bond. Organometallics 2020, 39, 4599–4607. [Google Scholar] [CrossRef]
- Sherstobitova, I.A.; KiselevaAlex, S.A.; Lyapkova, A.; Yusubova, M.S.; Zhdankinab, V.V.; Yu, B.-Y.; Verpoort., F. Synthesis and characterization of a novel latent ring-opening metathesis polymerization catalyst. Tetrahedron Lett. 2021, 84, 153451. [Google Scholar] [CrossRef]
- Öztürk, B.Ö.; SEhitolu, S.K.; Meier, M.A.R. A latent and Controllable Ruthenium-Indenylidene Catalyst for Emulsion ROMP in Water. Eur. Polym. J. 2015, 62, 116–123. [Google Scholar] [CrossRef]
- Richaud, A.; Barba-Behrens, N.; Méndez, F. Chemical Reactivity of the Imidazole: A Semblance of Pyridine and Pyrrole? Org. Lett. 2011, 13, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Butilkov, D.; Frenklah, A.; Rozenberg, I.; Kozuch, S.; Lemcoff, N.G. Highly Selective Olefin Metathesis with CAAC-Containing Ruthenium Benzylidenes. ACS Catal. 2017, 7, 7634–7637. [Google Scholar] [CrossRef]
- P’Pool, S.J.; Schanz, H. Reversible Inhibition/Activation of Olefin Metathesis: A Kinetic Investigation of ROMP and RCM Reactions with Grubbs’ Catalyst. J. Am. Chem. Soc. 2007, 129, 14200–14212. [Google Scholar]
- Dunbar, M.A.; Balof, S.L.; LaBeaud, L.J.; Yu, B.; Lowe, A.B.; Valente, E.J.; Schanz, H. Improved Molecular Weight Control in Ring-Opening Metathesis Polymerization (ROMP) Reactions with Ru-Based Olefin Metathesis Catalysts Using N Donors and Acid: A Kinetic and Mechanistic Investigation. Chem. Eur. J. 2009, 15, 12435–12446. [Google Scholar] [CrossRef]
- Öztürk, B.Ö.; Sariaslan, B.; Bayramgil, N.P.; SEhitolu, S.K. Highly Controllable Poly(N-vinylimidazole)-Supported Ruthenium Catalysts for Olefin Metathesis Reactions in Aqueous Media. Appl. Catal. A-Gen. 2014, 483, 19–24. [Google Scholar] [CrossRef]
- Sauvage, X.; Borguet, Y.; Noels, A.F.; Delaude, L.; Demonceau, A. Homobimetallic ruthenium-N-heterocyclic Carbene Complexes: Synthesis, Characterization, and Catalytic Applications. Adv. Synth. Catal. 2007, 349, 255–265. [Google Scholar] [CrossRef]
- Lo, C.; Cariou, R.; Fischmeister, C.; Dixneuf, P.H. Simple Ruthenium Precatalyst for the Synthesis of Stilbene Derivatives and Ring-Closing Metathesis in the Presence of Styrene Initiators. Adv. Synth. Catal. 2007, 349, 546–550. [Google Scholar] [CrossRef]
- Penn, K.R.; Anders, E.J.; Lindsay, V.N.G. Expedient Synthesis of Bis(imidazolium) Dichloride Salts and Bis(NHC) Complexes from Imidazoles Using DMSO as a Key Polar Additive. Organometallics 2021, 40, 3871–3875. [Google Scholar] [CrossRef]





| Entry | [Ru] | Temp (°C) | Time (h) | Additive | Solvent | Conv. (%) | Yield of 8 | Yield of 9 |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 25 | 4 | - | DCM | >99 | 97 | - |
| 2 | 6b | 25 | 4 | - | DCM | 31 | 13 | 15 |
| 3 b | 6b | 25 | 12 | HCl | DCM | >99 | 9 | 86 |
| 4 b | 6b | 25 | 12 | H3PO4 | DCM | >99 | 40 | 56 |
| 5 b | Schanz | 25 | 12 | HCl | DCM | >99 | Trace | 92 |
| 6 b | Schanz | 25 | 12 | H3PO4 | DCM | 96 | 80 | 11 |
| 7 | 6b | 40 | 4 | - | DCM | 45 | 21 | 23 |
| 8 | 6b | 60 | 4 | - | Toluene | 61 | 26 | 31 |
| 9 | 6b | 70 | 4 | - | Toluene | 73 | 33 | 38 |
| 10 | 6b | 80 | 4 | - | Toluene | >99 | 43 | 52 |
| 11 | 6b | 80 | 4 | phenylacetylene | Toluene | 91 | 14 | 73 |
| 12 | 6b | 80 | 4 | 3-bromoprop-1-yne | Toluene | 39 | 36 | - |
| 13 | 6b | 80 | 12 | 3-bromoprop-1-yne | Toluene | >99 | 95 | - |
| 14 | 6b | 80 | 8 | 3-bromoprop-1-yne | Toluene | 70 | 66 | - |
| 15 c | 6b | 80 | 12 | 3-bromoprop-1-yne | Toluene | 95 | 89 | - |
| 16 d | 6b | 80 | 12 | 3-bromoprop-1-yne | Toluene | >99 | 95 | - |
| 17 | 6b | 110 | 12 | 3-bromoprop-1-yne | Toluene | 79 | 70 | - |
| Entry | Substrate | Product | [Ru] | Yield |
|---|---|---|---|---|
| 1 |  |  | 6a | 93 |
| 6b | 95 | |||
| 2 |  |  | 6a | 85 |
| 6b | 89 | |||
| 3 |  |  | 6a | 82 |
| 6b | 88 | |||
| 4 |  |  | 6a | 86 |
| 6b | 89 | |||
| 5 |  |  | 6a | 0 |
| 6b | 0 | |||
| 6 |  |  | 6a | 82 |
| 6b | 87 | |||
| 7 |  |  | 6a | 80 |
| 6b | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, P.; Zhang, J.; Wu, X.; Wang, J. Ruthenium Metathesis Catalysts with Imidazole Ligands. Catalysts 2023, 13, 276. https://doi.org/10.3390/catal13020276
Ma P, Zhang J, Wu X, Wang J. Ruthenium Metathesis Catalysts with Imidazole Ligands. Catalysts. 2023; 13(2):276. https://doi.org/10.3390/catal13020276
Chicago/Turabian StyleMa, Peng, Jiaren Zhang, Xiaqian Wu, and Jianhui Wang. 2023. "Ruthenium Metathesis Catalysts with Imidazole Ligands" Catalysts 13, no. 2: 276. https://doi.org/10.3390/catal13020276