Single-Atom Iron Catalyst Based on Functionalized Mesophase Pitch Exhibiting Efficient Oxygen Reduction Reaction Activity
Abstract
:1. Introduction
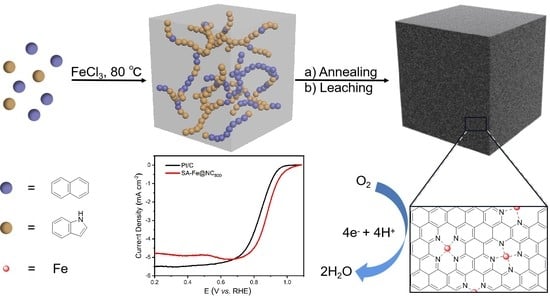
2. Results and Discussion
3. Materials and Methods
3.1. Negative ESI FT-ICR MS Characterization
3.2. Positive APPI FT-ICR MS Characterization
3.3. Electrochemical Measurement
3.4. Preparation of SA-Fe@NC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, L.A.W.; Hung, C.R.; Majeau-Bettez, G.; Singh, B.; Chen, Z.; Whittingham, M.S.; Strømman, A.H. Nanotechnology for environmentally sustainable electromobility. Nat. Nanotechnol. 2016, 486, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Waje, M.; Li, W.; Yan, Y. Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions. Angew. Chem. Int. Ed. 2007, 46, 4060–4063. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Bezerra, C.W.; Zhang, L.; Lee, K.; Liu, H.J.; Marques, A.L.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochem. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Nabae, Y.; Nagata, S.; Kusaba, K.; Aoki, T.; Hayakawa, T.; Tanida, H.; Imai, H.; Hori, K.; Yamamoto, Y.; Arai, S.; et al. Magnetic purification of non-precious metal fuel cell catalysts for obtaining atomically dispersed Fe centers. Catal. Sci. Technol. 2020, 10, 493–501. [Google Scholar] [CrossRef]
- Nabae, Y.; Sonoda, M.; Yamauchi, C.; Hosaka, Y.; Isoda, A.; Aoki, T. Highly durable Pt-free fuel cell catalysts prepared by multi-step pyrolysis of Fe phthalocyanine and phenolic resin. Catal. Sci. Technol. 2014, 4, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Single-atom dispersed Co–N–C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 2016, 128, 10958–10963. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef] [Green Version]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Li, J.; Xue, M.; Xu, N.; Zhang, X.; Wang, Y.; He, R.; Huang, H.; Qiao, J. Co/Ni dual-metal embedded in heteroatom doped porous carbon core-shell bifunctional electrocatalyst for rechargeable Zn-air batteries. Mater. Rep. Energy 2022, 2, 100090. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3–δ Perovskite in Energy Storage and Conversion: Past, Present, and Future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef]
- Sahraie, N.R.; Kramm, U.I.; Steinberg, J.; Zhang, Y.; Thomas, A.; Reier, T.; Paraknowitsch, J.-P.; Strasser, P. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 2015, 6, 8618. [Google Scholar] [CrossRef] [Green Version]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H.; et al. General synthesis and definitive structural identification of MN 4 C 4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun 2016, 7, 13285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Cheng, D.; Xu, H.; Zeng, X.; Wan, X.; Shui, J.; Xiang, Z.; Cao, D. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. USA 2018, 115, 6626–6631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, X.; Zheng, L.; Shang, J.; Wan, X.; Hu, R.; Guo, X.; Hong, S.; Shui, J. Preparation of Fe–N–C catalysts with FeN x (x= 1, 3, 4) active sites and comparison of their activities for the oxygen reduction reaction and performances in proton exchange membrane fuel cells. J. Mater. Chem. 2019, 7, 26147–26153. [Google Scholar] [CrossRef]
- Wen, Y.; Ma, C.; Wei, Z.; Zhu, X.; Li, Z. FeNC/MXene hybrid nanosheet as an efficient electrocatalyst for oxygen reduction reaction. RSC Adv. 2019, 9, 13424–13430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Sun, J.; Wang, F.; Dai, L. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew. Chem. Int. Ed. 2018, 130, 9176–9181. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-metal-free Fe–N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Song, J.; Shi, Q.; Su, D.; Engelhard, M.H.; Li, X.; Xiao, D.; Li, D.; Estevez, L.; et al. Self-Assembled Fe-N-Doped Carbon Nanotube Aerogels with Single-Atom Catalyst Feature as High-Efficiency Oxygen Reduction Electrocatalysts. Small 2017, 13, 16004–16007. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Li, Z.; Lv, L.; Ruan, Y.; Wu, H.H.; Chiang, W.H.; Wang, C.; Liu, M.; Zeng, X.C. Unraveling the high-activity nature of Fe-N-C electrocatalysts for the oxygen reduction reaction: The extraordinary synergy between Fe-N4 and Fe4-N. J. Mater. Chem. A 2019, 7, 11792–11801. [Google Scholar] [CrossRef]
- Wang, X.; Fang, J.; Liu, X.; Zhang, X.; Lv, Q.; Xu, Z.; Zhang, X.; Zhu, W.; Zhuang, Z. Converting biomass into efficient oxygen reduction reaction catalysts for proton exchange membrane fuel cells. Sci. China Mater. 2020, 63, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Song, X.; Zhao, C.; Hu, D.; Zhang, W.; Jia, M. N-Doped Porous Carbon–Fe x C Nanoparticle Composites as Catalysts for Friedel–Crafts Acylation. ACS Appl. 2020, 3, 6664–6674. [Google Scholar]
- Xiao, M.; Zhu, J.; Ma, L.; Jin, Z.; Ge, J.; Deng, X.; Hou, Y.; He, Q.; Li, J.; Jia, Q.; et al. Microporous framework induced synthesis of single-atom dispersed Fe-NC acidic ORR catalyst and its in situ reduced Fe-N4 active site identification revealed by X-ray absorption spectroscopy. ACS Catal. 2018, 8, 2824–2832. [Google Scholar] [CrossRef]
- Qiao, Y.; Yuan, P.; Hu, Y.; Zhang, J.; Mu, S.; Zhou, J.; Li, H.; Xia, H.; He, J.; Xu, Q. Sulfuration of an Fe–N–C catalyst containing FexC/Fe species to enhance the catalysis of oxygen reduction in acidic media and for use in flexible Zn–air batteries. Adv. Mater. 2018, 30, 1804504. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2017, 129, 7041–7045. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.G.; Chen, W.; Dong, J.; Zheng, L.; Luo, J.; Wan, J.; Tian, S.; Cheong, W.C.; Wang, D.; et al. Metal (hydr) oxides@ polymer core–shell strategy to metal single-atom materials. J. Am. Chem. Soc. 2017, 139, 10976–10979. [Google Scholar] [CrossRef]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1, e1400129. [Google Scholar] [CrossRef] [Green Version]
- Prieto, R.; Louis, E.; Molina, J. Fabrication of mesophase pitch-derived open-pore carbon foams by replication processing. Carbon 2012, 50, 1904–1912. [Google Scholar] [CrossRef]
- Alway-Cooper, R.M.; Anderson, D.P.; Ogale, A.A. Carbon black modification of mesophase pitch-based carbon fibers. Carbon 2013, 59, 40–48. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Du, H.; Li, Q.; Hou, X.; Ye, J. Preparation of mesophase pitch by aromatics-rich distillate of naphthenic vacuum gas oil. Appl. Petrochem. Res. 2015, 5, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Lou, B.; Li, M.; Qu, F.; Yu, R.; Yang, Y.; Wu, C. Study on the preparation of mesophase pitch from modified naphthenic vacuum residue by direct thermal treatment. Energy Fuels 2016, 30, 4609–4618. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, H.; Xu, H.; Westwood, A.; Li, X. Spinnable mesophase pitch prepared via co-carbonization of fluid catalytic cracking decant oil and synthetic naphthalene pitch. Energy Fuels 2019, 34, 2566–2573. [Google Scholar] [CrossRef]
- Minato, H.; Higosaki, N.; Isobe, C. Polymerization of Naphthalene and Reactions of Polynaphthalene. Bull. Chem. Soc. Jpn. 1969, 42, 779–781. [Google Scholar] [CrossRef]
- Korai, Y.; Mochida, I. Molecular assembly of mesophase and isotropic pitches at their fused states. Carbon 1992, 30, 1019–1024. [Google Scholar] [CrossRef]
- Mushrush, G.W.; Beal, E.J.; Hardy, D.R.; Hughes, J.M. Nitrogen compound distribution in middle distillate fuels derived from petroleum, oil shale, and tar sand sources. Fuel Process. Technol. 1999, 61, 197–210. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Sun, Z.; Zhang, D.; Huang, Y.; Gu, S.; Chen, W. Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon. Chemosphere 2020, 241, 125120. [Google Scholar] [CrossRef]
- Abthagir, P.S.; Dhanalakshmi, K.; Saraswathi, R. Thermal studies on polyindole and polycarbazole. Synth. Met. 1998, 93, 1–7. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of multitudinous active sites for oxygen reduction reaction in transition metal–nitrogen–carbon electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Tylus, U.; Jia, Q.; Mukerjee, S. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: Linking surface science to coordination chemistry. J. Am. Chem. Soc. 2013, 135, 15443–15449. [Google Scholar] [CrossRef]
- Jia, Q.; Ramaswamy, N.; Hafiz, H.; Tylus, U.; Strickland, K.; Wu, G.; Barbiellini, B.; Bansil, A.; Holby, E.F.; Zelenay, P.; et al. Experimental Observation of Redox-Induced Fe–N Switching Behavior as a Determinant Role for Oxygen Reduction Activity. ACS Nano 2015, 9, 12496–12505. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Wang, M.; Peng, H.; Peng, Q.; Wang, W.; Wang, H.; Shi, J.; Qin, X.; Da, Z.; Yang, W.; et al. Single-Atom Iron Catalyst Based on Functionalized Mesophase Pitch Exhibiting Efficient Oxygen Reduction Reaction Activity. Catalysts 2022, 12, 1608. https://doi.org/10.3390/catal12121608
Gu X, Wang M, Peng H, Peng Q, Wang W, Wang H, Shi J, Qin X, Da Z, Yang W, et al. Single-Atom Iron Catalyst Based on Functionalized Mesophase Pitch Exhibiting Efficient Oxygen Reduction Reaction Activity. Catalysts. 2022; 12(12):1608. https://doi.org/10.3390/catal12121608
Chicago/Turabian StyleGu, Xianrui, Meng Wang, Hongpeng Peng, Qian Peng, Wei Wang, Houpeng Wang, Junjun Shi, Xuetao Qin, Zhijian Da, Wenhong Yang, and et al. 2022. "Single-Atom Iron Catalyst Based on Functionalized Mesophase Pitch Exhibiting Efficient Oxygen Reduction Reaction Activity" Catalysts 12, no. 12: 1608. https://doi.org/10.3390/catal12121608