Selective C-O Coupling Reaction of N-Methoxy Arylamides and Arylboronic Acids Catalyzed by Copper Salt
Abstract
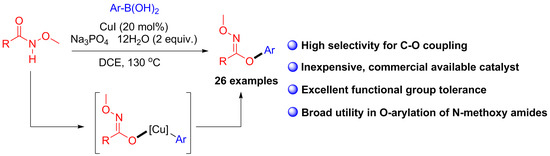
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Copper-Catalyzed C-O Coupling of N-Methoxy Amides
3.3. Characterization Data for Products 3a–3v
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheung, C.W.; Ploeger, M.L.; Hu, X. Direct amidation of esters with nitroarenes. Nat. Commun. 2017, 8, 14878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hili, R.; Yudin, A.K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2006, 2, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Szostak, M. Highly selective transition-metal-free transamidation of amides and amidation of esters at room temperature. Nat. Commun. 2018, 9, 4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, T.S.; Kulkarni, S.S.; Hossain, K.R.; Cornelius, F.; Clarke, R.J.; Payne, R.J. Peptide ligation at high dilution via reductive diselenide-selenoester ligation. J. Am. Chem. Soc. 2020, 142, 1090–1100. [Google Scholar] [CrossRef]
- Forero-Cortés, P.A.; Haydl, A.M. The 25th Anniversary of the Buchwald-Hartwig amination: Development, applications, and outlook. Org. Process Res. Dev. 2019, 23, 1478–1483. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blackera, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Meng, T.; Zhang, Y.L.; Li, M.; Wang, X.; Shen, J.K. Synthesis of novel substituted benzimidazo[1,2-a]quinoxalin-6(5H)-ones via an intramolecular Goldberg reaction. J. Comb. Chem. 2010, 12, 222–224. [Google Scholar] [CrossRef]
- Meng, G.R.; Szostak, M. Palladium-catalyzed Suzuki-Miyaura coupling of amides by carbon-nitrogen cleavage: General strategy for amide N-C bond activation. Org. Biomol. Chem. 2016, 14, 5690–5707. [Google Scholar] [CrossRef]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-coupling of aromatic esters and amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef]
- Wang, C.L.; Bai, X.; Wang, R.; Zheng, X.D.; Ma, X.M.; Chen, H.; Ai, Y.; Bai, Y.J.; Liu, Y.F. Synthesis of Imatinib by C-N coupling reaction of primary amide and bromo-substituted pyrimidine amine. Org. Process Res. Dev. 2019, 23, 1918–1925. [Google Scholar] [CrossRef]
- Chan, D.M.T.; Monaco, K.L.; Wang, R.-P.; Winters, M.P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 1998, 39, 2933–2936. [Google Scholar] [CrossRef]
- Alapati, M.L.P.R.; Abburu, S.R.; Mutyala, K.R.; Mukkamala, S.B. Copper (I) iodide-catalyzed amidation of phenylboronic acids/arylbromides using 4-dimethylaminopyridine as ligand. Synth. Commun. 2016, 46, 1242–1248. [Google Scholar] [CrossRef]
- Sahoo, H.; Mukherjee, S.; Grandhi, G.S.; Selvakumar, J.; Baidya, M. Copper catalyzed C-N cross-coupling reaction of arylboronic acids at room temperature through chelation assistance. J. Org. Chem. 2017, 82, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Roscalesa, S.; Csáky, A.G. How to make C-N bonds using boronic acids and their derivatives without transition metals. Chem. Soc. Rev. 2020, 49, 5159–5177. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Buchspies, J.; Szostak, M. N-Acylphthalimides: Efficient acyl coupling reagents in Suzuki-Miyaura cross-coupling by N-C cleavage catalyzed by Pd-PEPPSI precatalysts. Catalysts 2019, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Buchspies, J.; Rahman, M.M.; Szostak, M. Suzuki-Miyaura cross-coupling of amides using well-defined, air- and moisture-stable nickel/NHC (NHC = N-heterocyclic carbene) complexes. Catalysts 2020, 10, 372. [Google Scholar] [CrossRef] [Green Version]
- Lei, P.; Meng, G.; Szostak, M. General method for the Suzuki-Miyaura cross-coupling of amides using commercially available, air- and moisture-stable palladium/NHC (NHC = N-heterocyclic carbene) complexes. ACS Catal. 2017, 7, 1960–1965. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. Acyl and Decarbonylative Suzuki coupling of N-acetyl amides: Electronic tuning of twisted, acyclic amides in catalytic carbon−nitrogen bond cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar] [CrossRef]
- Osumi, Y.; Liu, C.; Szostak, M. N-Acylsuccinimides: Twist-controlled, acyl-transfer reagents in Suzuki-Miyaura cross-coupling by N-C amide bond activation. Org. Biomol. Chem. 2017, 15, 8867–8871. [Google Scholar] [CrossRef]
- Szostak, M.; Meng, G.; Shi, S. Palladium-catalyzed Suzuki−Miyaura cross-coupling of amides via site-selective N−C bond cleavage by cooperative catalysis. ACS Catal. 2016, 6, 7335–7339. [Google Scholar]
- Meng, G.; Szostak, R.; Szostak, M. Suzuki−Miyaura cross-coupling of N-acylpyrroles and pyrazoles: Planar, electronically activated amides in catalytic N-C cleavage. Org. Lett. 2017, 19, 3596–3599. [Google Scholar] [CrossRef] [PubMed]
- Racine, E.; Monnier, F.; Vors, J.-P.; Taillefer, M. Direct N-cyclopropylation of secondary acyclic amides promoted by copper. Chem. Commun. 2013, 49, 7412–7414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.A.; Shimkin, K.W.; Xu, Q.; Mori-Quiroz, L.M.; Watson, D.A. Selective formation of secondary amides via the copper-catalyzed cross-coupling of alkylboronic acids with primary amides. Org. Lett. 2013, 15, 2314–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Sarma, M.J.; Kashyap, B.; Phukan, P. A quick Chan-Lam C-N and C-S cross coupling at room temperature in the presence of square pyramidal [Cu(DMAP)4I]I as a catalyst. Chem. Commun. 2016, 52, 1170–1173. [Google Scholar] [CrossRef]
- Munir, I.; Zahoor, A.F.; Rasool, N.; Naqvi, S.A.R.; Zia, K.M.; Ahmad, R. Synthetic applications and methodology development of Chan-Lam coupling: A review. Mol. Divers. 2019, 23, 215–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Liebeskind, L.S. N-amidation by copper-mediated cross-coupling of organostannanes or boronic acids with O-acetyl hydroxamic Acids. Org. Lett. 2008, 10, 3005–3008. [Google Scholar] [CrossRef] [Green Version]
- Broumidis, E.; Jones, M.C.; Vilela, F.; Lloyd, G.O. Mechanochemical synthesis of N-aryl amides from O-protected hydroxamic acids. ChemPlusChem 2020, 85, 1754–1761. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, Y.; Xie, H.L.; Ren, S.F.; Liu, J.-B.; Luo, N.H.; Qiu, G.Y.S. Iron-catalyzed cross-coupling of N-methoxy amides and arylboronic acids for the synthesis of N-aryl amides. Mol. Catal. 2021, 516, 111993. [Google Scholar] [CrossRef]
- Lai, X.J.; Liu, J.-B.; Wang, Y.C.; Qiu, G.Y.S. Iron-catalyzed intramolecular acyl nitrene/alkyne metalation for the synthesis of pyrrolo[2,1-a]isoindol-5-ones. Chem. Commun. 2021, 57, 2077–2080. [Google Scholar] [CrossRef]
- Liu, J.-B.; Ren, M.F.; Lai, X.J.; Qiu, G.Y.S. Iron-catalyzed stereoselective haloamidation of amide-tethered alkynes. Chem. Commun. 2021, 57, 4259–4262. [Google Scholar] [CrossRef]
- Zhong, P.Y.; Wu, J.J.; Wu, J.R.; Liu, K.M.; Wang, C.F.; Liu, J.-B. Solvent-controlled selective synthesis of amides and thioureas from isothiocyanates. Tetrahedron Lett. 2022, 107, 154099. [Google Scholar] [CrossRef]
- Chen, Z.H.; Hu, L.A.; Zeng, F.Y.; Zhu, R.R.; Yu, Q.Z.; Huang, J.H. Selective mono-alkylation of N-methoxybenzamides. Chem. Commun. 2017, 53, 4258–4261. [Google Scholar] [CrossRef]
- Rao, W.-H.; Jiang, L.-L.; Zhao, J.-X.; Jiang, X.; Zou, G.-D.; Zhou, Y.-Q.; Tang, L. Selective O-cyclization of N-methoxy aryl amides with CH2Br2 or 1,2-DCE via palladium-catalyzed C-H activation. Org. Lett. 2018, 20, 6198–6201. [Google Scholar] [CrossRef]
- Deng, X.M.; Wang, Y.; Liu, J.-B.; Wan, C.F.; Luo, N.H. Synthesis of N-methoxy-1 phosphoryloxy imidates through a copper-catalyzed cross-dehydrogenative coupling of N-methoxylamides with phosphites. Tetrahedron Lett. 2022, 105, 154049. [Google Scholar] [CrossRef]
- Collman, J.P.; Zhong, M.; Zhang, C.; Costanzo, S. Catalytic activities of Cu(II) complexes with nitrogen-chelating bidentate ligands in the coupling of imidazoles with arylboronic acids. J. Org. Chem. 2001, 66, 7892. [Google Scholar] [CrossRef]
- Wang, R.X.; Xie, H.L.; Lai, X.J.; Liu, J.-B.; Li, J.H.; Qiu, G.Y.S. Visible light-enabled iron-catalyzed selenocyclization of N-methoxy-2-alkynylbenzamide. Mol. Catal. 2021, 515, 111881. [Google Scholar] [CrossRef]
- Ren, M.F.; Yan, X.Y.; Lai, X.J.; Liu, J.-B.; Zhou, H.W.; Qiu, G.Y.S. Nitrenium ion-based ipso-addition and ortho-cyclization of arenes under photo and iron dual-catalysis. Mol. Catal. 2022, 528, 112413. [Google Scholar] [CrossRef]






| Entry | [Cu] | Base (equiv) | Solv. | Temp. (°C) | Yield of 3a b (%) | Yield of 4a b (%) |
| 1 | Cu(OAc)2·H2O | K2CO3 | DCE | 80 | 28 | 6 |
| 2 | Cu(OAc)2·H2O | - | DCE | RT | ˂5 | 9 |
| 3 | Cu(OAc)2·H2O | K2CO3 | DCE | RT | 30 | 19 |
| 4 | Cu(OAc)2·H2O | Et3N | DCE | RT | ˂5 | 12 |
| 5 | Cu(OAc)2·H2O | KOH | DCE | RT | 18 | 11 |
| 6 | Cu(OAc)2·H2O | Cs2CO3 | DCE | RT | 19 | 30 |
| 7 | Cu(OAc)2·H2O | t-BuOK | DCE | RT | 11 | 33 |
| 8 | Cu(OAc)2·H2O | Na2CO3 | DCE | RT | 8 | 31 |
| 9 | Cu(OAc)2·H2O | Na3PO4·12H2O | DCE | RT | 34 | 29 |
| 10 | Cu(OAc)2·H2O | Na3PO4·12H2O | THF | RT | 0 | 0 |
| 11 | Cu(OAc)2·H2O | Na3PO4·12H2O | acetone | RT | ˂5 | ˂5 |
| 12 | Cu(OAc)2·H2O | Na3PO4·12H2O | EA | RT | 22 | ˂5 |
| 13 | Cu(OAc)2·H2O | Na3PO4·12H2O | EtOH | RT | 0 | ˂5 |
| 14 | Cu(OAc)2·H2O | Na3PO4·12H2O | Toluene | RT | 32 | 25 |
| 15 | Cu(OAc)2·H2O | Na3PO4·12H2O | DMF | RT | 0 | 0 |
| 16 | Cu(OAc)2·H2O | Na3PO4·12H2O | 1,4-dioxane | RT | 0 | 0 |
| 17 | Cu(OAc)2·H2O | Na3PO4·12H2O | DMSO | RT | 0 | 0 |
| 18 | Cu(OAc)2·H2O | Na3PO4·12H2O | Et2O | RT | 31 | 9 |
| 19 | Cu(OAc)2·H2O | Na3PO4·12H2O | MeCN | RT | ˂5 | ˂5 |
| 20 | Cu(OAc)2·H2O | Na3PO4·12H2O | DCE | 40 | 18 | ˂5 |
| 21 | Cu(OAc)2·H2O | Na3PO4·12H2O | DCE | 80 | 28 | ˂5 |
| 22 | Cu(OAc)2·H2O | Na3PO4·12H2O | DCE | 120 | 41 | ˂5 |
| 23 | Cu(OAc)2·H2O | Na3PO4·12H2O | DCE | 130 | 48 | ˂5 |
| 24 | Cu(OAc)2·H2O | Na3PO4·12H2O | DCE | 140 | 30 | 0 |
| 25 | CuI | Na3PO4·12H2O | DCE | 130 | 70 | 0 |
| 26 | CuCl2 | Na3PO4·12H2O | DCE | 130 | 38 | 16 |
| 27 | CuBr | Na3PO4·12H2O | DCE | 130 | 37 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, H.; Liu, K.; Li, J.; Liu, J.-B. Selective C-O Coupling Reaction of N-Methoxy Arylamides and Arylboronic Acids Catalyzed by Copper Salt. Catalysts 2022, 12, 1278. https://doi.org/10.3390/catal12101278
Wang Y, Xie H, Liu K, Li J, Liu J-B. Selective C-O Coupling Reaction of N-Methoxy Arylamides and Arylboronic Acids Catalyzed by Copper Salt. Catalysts. 2022; 12(10):1278. https://doi.org/10.3390/catal12101278
Chicago/Turabian StyleWang, Ying, Huilin Xie, Kunming Liu, Jinhui Li, and Jin-Biao Liu. 2022. "Selective C-O Coupling Reaction of N-Methoxy Arylamides and Arylboronic Acids Catalyzed by Copper Salt" Catalysts 12, no. 10: 1278. https://doi.org/10.3390/catal12101278