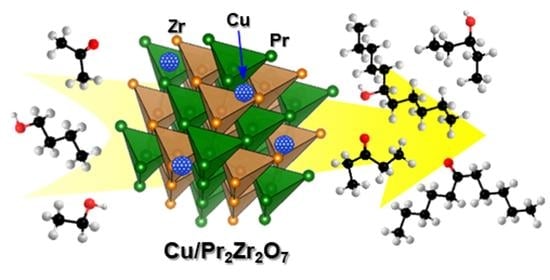
Efficient Cross-Coupling of Acetone with Linear Aliphatic Alcohols over Supported Copper on a Fluorite-Type Pr2Zr2O7
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Supported Cu Catalysts
2.2. Activity and Stability Comparison of Supported Cu Catalysts
2.3. Catalytic Performance of Cu/Pr2Zr2O7 with Different Cu Loadings
2.4. Catalytic Performance of Cu/Pr2Zr2O7 with Respect to the Reaction Time and H2O Content
2.5. Catalyst Recyclability of Cu/Pr2Zr2O7
2.6. Catalytic Performance of Cu/Pr2Zr2O7 in the Cross-Coupling of the ABE Mixture
3. Materials and Methods
3.1. Preparation of Supported Cu Catalysts
3.2. Catalyst Characterization
3.3. Activity Tests for the Cross-Coupling of Acetone with Butanol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N., Jr.; Ferreira, T. Clostridium sp. as bio-catalyst for fuels and chemicals production in a biorefinery context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Maddox, I.S. The acetone–butanol–ethanol fermentation: Recent progress in technology. Biotechnol. Genet. Eng. Rev. 1989, 7, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Jang, Y.S.; Moon, H.G.; Lee, J.; Lee, S.Y. Metabolic engineering of clostridia for the production of chemicals. Biofuels Bioprod. Bioref. 2015, 9, 211–225. [Google Scholar] [CrossRef]
- Moon, H.G.; Jang, Y.-S.; Cho, C.; Lee, J.; Binkley, R.; Lee, S.Y. One hundred years of clostridial butanol fermentation. FEMS Microbiol. Lett. 2016, 363, fnw001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbarasan, P.; Baer, Z.C.; Sreekumar, S.; Gross, E.; Binder, J.B.; Blanch, H.W.; Clark, D.S.; Toste, F.D. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 2012, 491, 235–239. [Google Scholar] [CrossRef]
- Sreekumar, S.; Baer, Z.C.; Gross, E.; Padmanaban, S.; Goulas, K.; Gunbas, G.; Alayoglu, S.; Blanch, H.W.; Clark, D.S.; Toste, F.D. Chemocatalytic upgrading of tailored fermentation products toward biodiesel. ChemSusChem 2014, 7, 2445–2448. [Google Scholar] [CrossRef]
- Xu, G.; Li, Q.; Feng, J.; Liu, Q.; Zhang, Z.; Wang, X.; Zhang, X.; Mu, X. Direct α-alkylation of ketones with alcohols in water. ChemSusChem 2014, 7, 105–109. [Google Scholar] [CrossRef]
- Zhu, Q.; Shen, C.; Wang, J.; Tan, T. Upgrade of solvent-free acetone–butanol–ethanol mixture to high-value biofuels over Ni-containing MgO–SiO2 catalysts with greatly improved water-resistance. ACS Sustain. Chem. Eng. 2017, 5, 8181–8191. [Google Scholar] [CrossRef]
- Fridrich, B.; Stuart, M.C.A.; Barta, K. Selective coupling of bioderived aliphatic alcohols with acetone using hydrotalcite derived Mg−Al porous metal oxide and Raney nickel. ACS Sustain. Chem. Eng. 2018, 6, 8468–8475. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Kannapu, H.P.R.; Suh, Y.-W. Cross-aldol condensation of acetone and n-butanol into aliphatic ketones over supported Cu catalysts on ceria-zirconia. Catalysts 2017, 7, 249. [Google Scholar] [CrossRef] [Green Version]
- Kannapu, H.P.R.; Kim, M.; Jeong, C.; Suh, Y.-W. An efficient Cu–CeO2 citrate catalyst for higher aliphatic ketone synthesis via alkali-free alkylation of acetone with butanol. Mater. Chem. Phys. 2019, 229, 402–411. [Google Scholar] [CrossRef]
- Itoh, M.; Motoki, K.; Saito, M.; Iwamoto, J.; Machida, K. Lean NOx reduction by hydrogen over Pt-supported rare earth oxide catalysts and their in situ DRIFTs study. Bull. Chem. Soc. Jpn. 2009, 82, 1197–1202. [Google Scholar] [CrossRef]
- Maitra, A.M. Determination of solid state basicity of rate earth oxides by thermal analysis of their carbonates. J. Therm. Anal. 1990, 36, 657–675. [Google Scholar] [CrossRef]
- Alcalde-Santiago, V.; Bailón-García, E.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Three-dimensionally ordered macroporous PrOx: An improved alternative to ceria catalysts for soot combustion. Appl. Catal. B Environ. 2019, 248, 567–572. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Igarashi, A.; Inoue, H. Catalytic reaction of 1,3-butanediol over rare earth oxides. Appl. Catal. A Gen. 2007, 328, 109–116. [Google Scholar] [CrossRef]
- Borchert, Y.; Sonström, P.; Wilhelm, M.; Borchert, H.; Bäumer, M. Nanostructured praseodymium oxide: Preparation, structure, and catalytic properties. J. Phys. Chem. C 2008, 112, 3054–3063. [Google Scholar] [CrossRef]
- Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 2017, 8, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Narula, C.K.; Haack, L.P.; Chun, W.; Jen, H.-W.; Graham, G.W. Single-phase PrOy–ZrO2 materials and their oxygen storage capacity: A comparison with single-phase CeO2–ZrO2, PrOy–CeO2, and PrOy–CeO2–ZrO2 materials. J. Phys. Chem. B 1999, 103, 3634–3639. [Google Scholar] [CrossRef]
- Stefanik, T.S.; Tuller, H.L. Nonstoichiometry and defect chemistry in praseodymium-cerium oxide. J. Electroceram. 2004, 13, 799–803. [Google Scholar] [CrossRef]
- Borchert, H.; Frolova, Y.V.; Kaichev, V.V.; Prosvirin, I.P.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Trukhan, S.N.; Ivanov, V.P.; et al. Electronic and chemical properties of nanostructured cerium dioxide doped with praseodymium. J. Phys. Chem. B 2005, 109, 5728–5738. [Google Scholar] [CrossRef] [PubMed]
- Tankov, I.; Pawelec, B.; Arishtirova, K.; Damyanova, S. Structure and surface properties of praseodymium modified alumina. Appl. Surf. Sci. 2011, 258, 278–284. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Zhu, X.; Du, Y.; Wei, Y.; Zhai, K.; Wang, H. Synthesis of mesoporous PrxZr1−xO2−δ solid solution with high thermal stability for catalytic soot oxidation. J. Ind. Eng. Chem. 2017, 54, 126–136. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Zoccoli, M.; Giménez-Mañogil, J.; García-García, A. Lattice oxygen activity in ceria-praseodymia mixed oxides for soot oxidation in catalysed gasoline particle filters. Appl. Catal. B Environ. 2019, 248, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Yamamura, H.; Nishino, H.; Kakinuma, K.; Nomura, K. Crystal phase and electrical conductivity in the pyrochlore-type composition systems, Ln2Ce2O7 (Ln = La, Nd, Sm, Eu, Gd, Y and Yb). J. Ceram. Soc. Jpn. 2003, 111, 902–906. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, H.; Nishino, H.; Kakinuma, K. Ac conductivity for Eu2Zr2O7 and La2Ce2O7 with pyrochlore-type composition. J. Ceram. Soc. Jpn. 2004, 112, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Matović, B.; Maletaškić, J.; Yoshida, K.; Yano, T. Synthesis, characterization and sintering of fluorite and pyrochlore-type compounds: Pr2Zr2O7, Sm2Zr2O7 and PrSmZr2O7. Mater. Today Proc. 2019, 16, 156–162. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Han, X.; Wu, Y.; Liu, W.; Zhang, R.; Zhang, N.; Wang, X. Elucidating the promotional effects of niobia on SnO2 for CO oxidation: Developing an XRD extrapolation method to measure the lattice capacity of solid solutions. Catal. Sci. Technol. 2016, 6, 5280–5291. [Google Scholar] [CrossRef]
- Paunović, N.; Dohčević-Mitrović, Z.; Scurtu, R.; Aškrabić, S.; Prekajski, M.; Matović, B.; Popović, Z.V. Suppression of inherent ferromagnetism in Pr-doped CeO2 nanocrystals. Nanoscale 2012, 4, 5469–5476. [Google Scholar] [CrossRef]
- Kim, J.-S.; Na, C.W.; Kwak, C.-H.; Li, H.-Y.; Yoon, J.W.; Kim, J.-H.; Jeong, S.-Y.; Lee, J.-H. Humidity-independent gas sensors using Pr-doped In2O3 macroporous spheres: Role of cyclic Pr3+/Pr4+ redox reactions in suppression of water-poisoning effect. ACS Appl. Mater. Interfaces 2019, 11, 25322–25329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, X.; Feng, X.; Li, X.; Liu, W.; Xu, X.; Zhang, N.; Gao, Z.; Wang, X.; Zhou, W. Ni/Ln2Zr2O7 (Ln = La, Pr, Sm and Y) catalysts for methane steam reforming: The effects of A site replacement. Catal. Sci. Technol. 2017, 7, 2729–2743. [Google Scholar] [CrossRef]
- Al Kutubi, H.; Rassaei, L.; Olthuis, W.; Nelson, G.W.; Foord, J.S.; Holdway, P.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Tsang, S.C.; et al. Polymers of intrinsic microporosity as high temperature templates for the formation of nanofibrous oxides. RSC Adv. 2015, 5, 73323–73326. [Google Scholar] [CrossRef] [Green Version]
- Oemar, U.; Ang, M.L.; Chin, Y.C.; Hidajat, K.; Kawi, S. Role of lattice oxygen in oxidative steam reforming of toluene as a tar model compound over Ni/La0.8Sr0.2AlO3 catalyst. Catal. Sci. Technol. 2015, 5, 3585–3597. [Google Scholar] [CrossRef]
- Ahn, K.; Yoo, D.S.; Hari Prasa, D.; Lee, H.-W.; Chung, Y.-C.; Lee, J.-H. Role of multivalent Pr in the formation and migration of oxygen vacancy in Pr-doped ceria: Experimental and first-principles investigations. Chem. Mater. 2012, 24, 4261–4267. [Google Scholar] [CrossRef]
- Westermann, A.; Geantet, C.; Vernoux, P.; Loridant, S. Defects band enhanced by resonance Raman effect in praseodymium doped CeO2. J. Raman Spectrosc. 2016, 47, 1276–1279. [Google Scholar] [CrossRef]
- D’Angelo, A.M.; Chaffee, A.L. Correlations between oxygen uptake and vacancy concentration in Pr-doped CeO2. ACS Omega 2017, 2, 2544–2551. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Shen, C.; Wang, J.; Wu, C.; Tan, T. Improved selectivity of long-chain products from aqueous acetone–butanol–ethanol mixture over high water resistant catalyst based on hydrophobic SBA-16. ACS Sustain. Chem. Eng. 2019, 7, 10323–10331. [Google Scholar] [CrossRef]












| Catalyst | Cu loading a (wt %) | SBET b (m2 g–1) | Vpb (cm3 g–1) | SCu c (m2 g–1) | DCu c (%) | NCO2 d (μmol g–1) |
|---|---|---|---|---|---|---|
| Cu/m-ZrO2 | 5.75 | 22.4 | 0.07 | 3.9 | 30.4 | 37 (33) |
| Cu/PrO1.83 | 5.63 | 29.7 | 0.23 | 2.5 | 19.3 | 785 (418) |
| Cu/Pr2Zr2O7 | 5.61 | 13.6 | 0.03 | 2.0 | 15.8 | 187 (109) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Kim, M.; Ko, S.H.; Lee, J.-H.; Baik, J.H.; Suh, Y.-W. Efficient Cross-Coupling of Acetone with Linear Aliphatic Alcohols over Supported Copper on a Fluorite-Type Pr2Zr2O7. Catalysts 2022, 12, 1279. https://doi.org/10.3390/catal12101279
Lim S, Kim M, Ko SH, Lee J-H, Baik JH, Suh Y-W. Efficient Cross-Coupling of Acetone with Linear Aliphatic Alcohols over Supported Copper on a Fluorite-Type Pr2Zr2O7. Catalysts. 2022; 12(10):1279. https://doi.org/10.3390/catal12101279
Chicago/Turabian StyleLim, Suhyun, Minseok Kim, Sang Hyeok Ko, Jae-Hong Lee, Joon Hyun Baik, and Young-Woong Suh. 2022. "Efficient Cross-Coupling of Acetone with Linear Aliphatic Alcohols over Supported Copper on a Fluorite-Type Pr2Zr2O7" Catalysts 12, no. 10: 1279. https://doi.org/10.3390/catal12101279