Functional Foods: A Promising Strategy for Restoring Gut Microbiota Diversity Impacted by SARS-CoV-2 Variants
Abstract
:1. Introduction
2. What Qualifies a Food as “Functional”?
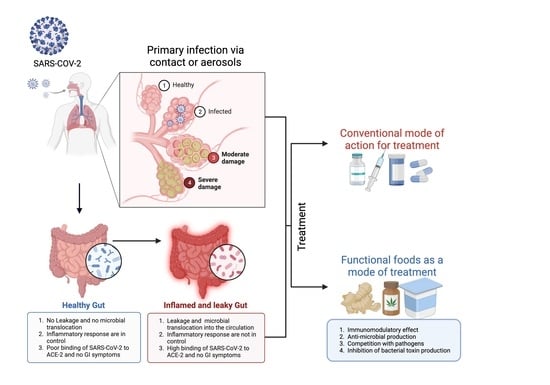
3. SARS-CoV-2 Infection in the Gastrointestinal Tract
3.1. Gastrointestinal Complications in COVID-19
3.2. Alteration of Gut Microbiota upon SARS-CoV-2 Infection
3.3. Loss of Gut Microbial Diversity and Associated Risk Factors in COVID-19 Patients
4. Therapies in the Management of Gut-Related Symptoms in COVID-19 Patients
4.1. Influence of Prebiotics and Probiotics on SARS-CoV-2 Infection
4.2. Probiotics Modulate Gut Microbiota
4.3. Natural Small Molecules and Functional Foods against COVID-19 Infection
5. Discussion and Conclusions
6. Study Highlights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, M.; Chatterji, S.; Gupta, S.K.; Watal, G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci. 2014, 6, 539–542. [Google Scholar]
- Farzana, M.; Shahriar, S.; Jeba, F.R.; Tabassum, T.; Araf, Y.; Ullah, M.A.; Tasnim, J.; Chakraborty, A.; Naima, T.A.; Marma, K.K.S. Functional food: Complementary to fight against COVID-19. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maathuis, F.J.; Diatloff, E. Roles and functions of plant mineral nutrients. Plant Miner. Nutr. Methods Protoc. 2013, 953, 1–21. [Google Scholar]
- Chadha, J.; Khullar, L.; Mittal, N. Facing the wrath of enigmatic mutations: A review on the emergence of severe acute respiratory syndrome coronavirus 2 variants amid coronavirus disease-19 pandemic. Environ. Microbiol. 2022, 24, 2615–2629. [Google Scholar] [CrossRef]
- Papanikolaou, V.; Chrysovergis, A.; Ragos, V.; Tsiambas, E.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Roukas, D.; Mastronikolis, S.; Peschos, D. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene 2022, 814, 146134. [Google Scholar] [CrossRef]
- Robson, F.; Khan, K.S.; Le, T.K.; Paris, C.; Demirbag, S.; Barfuss, P.; Rocchi, P.; Ng, W.-L. Coronavirus RNA proofreading: Molecular basis and therapeutic targeting. Mol. Cell 2020, 79, 710–727. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Kandi, V.; Tuli, H.S.; Chakraborty, C.; Dhama, K. The recombinant variants of SARS-CoV-2: Concerns continues amid COVID-19 pandemic. J. Med. Virol. 2022, 94, 3506. [Google Scholar] [CrossRef]
- Mercatelli, D.; Giorgi, F.M. Geographic and genomic distribution of SARS-CoV-2 mutations. Front. Microbiol. 2020, 11, 1800. [Google Scholar] [CrossRef]
- O’Toole, Á.; Pybus, O.G.; Abram, M.E.; Kelly, E.J.; Rambaut, A. Pango lineage designation and assignment using SARS-CoV-2 spike gene nucleotide sequences. BMC Genom. 2022, 23, 121. [Google Scholar] [CrossRef]
- Parums, D.V. Revised World Health Organization (WHO) terminology for variants of concern and variants of interest of SARS-CoV-2. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e933622-1. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042.e1021. [Google Scholar] [CrossRef]
- Alwaheed, A.J.; Alalwan, M.A.; Aldakhlan, H.M.; Albeladi, F.H. Necrotizing pancreatitis with portal vein thrombosis in young patient with COVID-19. J. Infect. Public Health 2022, 15, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kuang, D.; Li, D.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Cao, H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor Rev. 2022, 63, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.G.; Cuadrat, R.R.; Tonetti, F.R.; Kitazawa, H.; Villena, J. The role of respiratory microbiota in the protection against viral diseases: Respiratory commensal bacteria as next-generation probiotics for COVID-19. Biosci. Microbiota Food Health 2022, 41, 94–102. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in microbiota of patients with COVID-19: Potential mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 143. [Google Scholar] [CrossRef]
- Rajeev, R.; Seethalakshmi, P.; Jena, P.K.; Prathiviraj, R.; Kiran, G.S.; Selvin, J. Gut microbiome responses in the metabolism of human dietary components: Implications in health and homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 62, 7615–7631. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.S.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef]
- Cabinian, A.; Sinsimer, D.; Tang, M.; Jang, Y.; Choi, B.; Laouar, Y.; Laouar, A. Gut symbiotic microbes imprint intestinal immune cells with the innate receptor SLAMF4 which contributes to gut immune protection against enteric pathogens. Gut 2018, 67, 847–859. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, L.; Tarnow, I.; Eskesen, D.; Morberg, C.M.; Michelsen, B.; Dragsted, L.O.; Rijkers, G.T.; Calder, P.C. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Am. J. Clin. Nutr. 2015, 101, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezard, N.; Oppenheimer, E.; Burton, A.; Schilperoord, M.; Macdonald, D.; Adelekan, M.; Sakarati, A.; van Ommeren, M. Six rapid assessments of alcohol and other substance use in populations displaced by conflict. Confl. Health 2011, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, N.S.; Jukes, D.J. Functional foods. Part 1: The development of a regulatory concept. Food Control 2001, 12, 99–107. [Google Scholar] [CrossRef]
- Henry, C.J. Functional foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, D.M.; Singh, J. A new definition of functional food by FFC: What makes a new definition unique? Funct. Foods Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur. J. Med. Chem. 2022, 232, 114175. [Google Scholar] [CrossRef]
- Malone, B.; Urakova, N.; Snijder, E.J.; Campbell, E.A. Structures and functions of coronavirus replication–transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Mol. Cell Biol. 2022, 23, 21–39. [Google Scholar] [CrossRef]
- Chen, T.-H.; Hsu, M.-T.; Lee, M.-Y.; Chou, C.-K. Gastrointestinal involvement in SARS-CoV-2 infection. Viruses 2022, 14, 1188. [Google Scholar] [CrossRef]
- Baddock, H.T.; Brolih, S.; Yosaatmadja, Y.; Ratnaweera, M.; Bielinski, M.; Swift, L.P.; Cruz-Migoni, A.; Fan, H.; Keown, J.R.; Walker, A.P. Characterization of the SARS-CoV-2 ExoN (nsp14ExoN–nsp10) complex: Implications for its role in viral genome stability and inhibitor identification. Nucleic Acids Res. 2022, 50, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Revzin, M.V.; Raza, S.; Srivastava, N.C.; Warshawsky, R.; D’agostino, C.; Malhotra, A.; Bader, A.S.; Patel, R.D.; Chen, K.; Kyriakakos, C.; et al. Multisystem imaging manifestations of COVID-19, part 2: From cardiac complications to pediatric manifestations. Radiographics 2020, 40, 1866–1892. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S. Gastrointestinal Endoscopy in Patients with Coronavirus Disease 2019: Indications, Findings, and Safety. Gastroenterol. Clin. 2023, 52, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef]
- Lau, H.; Ng, S.; Yu, J. Targeting the gut microbiota in COVID-19: Hype or hope? Gastroenterology 2021, 162, 9–16. [Google Scholar] [CrossRef]
- Goguyer-Deschaumes, R.; Waeckel, L.; Killian, M.; Rochereau, N.; Paul, S. Metabolites and secretory immunoglobulins: Messengers and effectors of the host–microbiota intestinal equilibrium. Trends Immunol. 2022, 43, 63–77. [Google Scholar] [CrossRef]
- Posner, D.A.; Lee, C.Y.; Portet, A.; Clatworthy, M.R. Humoral immunity at the brain borders in homeostasis. Curr. Opin. Immunol. 2022, 76, 102188. [Google Scholar] [CrossRef]
- Craig, C.F.; Filippone, R.T.; Stavely, R.; Bornstein, J.C.; Apostolopoulos, V.; Nurgali, K. Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J. Neuroinflammation 2022, 19, 4. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Zuo, T.; Yeoh, Y.K.; Liu, Q.; Zhang, L.; Zhan, H.; Lu, W.; Xu, W.; Lui, G.C. Prolonged impairment of short-chain fatty acid and L-isoleucine biosynthesis in gut microbiome in patients with COVID-19. Gastroenterology 2022, 162, 548–561. e544. [Google Scholar] [CrossRef] [PubMed]
- Rasmi, Y.; Hatamkhani, S.; Naderi, R.; Shokati, A.; Zadeh, V.N.; Hosseinzadeh, F.; Farnamian, Y.; Jalali, L. Molecular signaling pathways, pathophysiological features in various organs, and treatment strategies in SARS-CoV2 infection. Acta Histochem. 2022, 124, 151908. [Google Scholar] [CrossRef] [PubMed]
- Farsi, Y.; Tahvildari, A.; Arbabi, M.; Vazife, F.; Sechi, L.A.; Shahidi Bonjar, A.H.; Jamshidi, P.; Nasiri, M.J.; Mirsaeidi, M. Diagnostic, prognostic, and therapeutic roles of gut microbiota in COVID-19: A comprehensive systematic review. Front. Cell. Infect. Microbiol. 2022, 12, 182. [Google Scholar] [CrossRef]
- Rastogi, S.; Mohanty, S.; Sharma, S.; Tripathi, P. Possible role of gut microbes and host’s immune response in gut–lung homeostasis. Front. Immunol. 2022, 13, 954339. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955.e948. [Google Scholar] [CrossRef] [PubMed]
- Reinold, J.; Farahpour, F.; Schoerding, A.-K.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; Van Baal, L.; Buer, J.; Witzke, O. The fungal gut microbiome exhibits reduced diversity and increased relative abundance of ascomycota in severe COVID-19 illness and distinct interconnected communities in SARS-CoV-2 positive patients. Front. Cell. Infect. Microbiol. 2022, 466, 848650. [Google Scholar] [CrossRef]
- Hazan, S.; Stollman, N.; Bozkurt, H.S.; Dave, S.; Papoutsis, A.J.; Daniels, J.; Barrows, B.D.; Quigley, E.M.; Borody, T.J. Lost microbes of COVID-19: Bifidobacterium, Faecalibacterium depletion and decreased microbiome diversity associated with SARS-CoV-2 infection severity. BMJ Open Gastroenterol. 2022, 9, e000871. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.F.; Marinkovic, A.; Prakash, S.; Sanyaolu, A.; Smith, S. COVID-19 and the Human Gut Microbiome: An Under-Recognized Association. Chonnam Med. J. 2022, 58, 96–101. [Google Scholar] [CrossRef]
- Hernández-Flores, T.d.J.; Pedraza-Brindis, E.J.; Cárdenas-Bedoya, J.; Ruíz-Carrillo, J.D.; Méndez-Clemente, A.S.; Martínez-Guzmán, M.A.; Iñiguez-Gutiérrez, L. Role of Micronutrients and Gut Microbiota-Derived Metabolites in COVID-19 Recovery. Int. J. Mol. Sci. 2022, 23, 12324. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, P.; Meng, W.; Jin, W.; Li, X.; Li, X. Modulated gut microbiota for potential COVID-19 prevention and treatment. Front. Med. 2022, 9, 811176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.; Lu, W.; Hui, D.S.-C. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Yaseen, Z.; Alanazi, M.; Alharby, T.N.; Alshammari, H.A.; Anwar, S. Association of SARS-CoV-2 and Polypharmacy with Gut–Lung Axis: From Pathogenesis to Treatment. ACS Omega 2022, 7, 33651–33665. [Google Scholar] [CrossRef] [PubMed]
- Baksh, R.A.; Strydom, A.; Pape, S.E.; Chan, L.F.; Gulliford, M.C. Susceptibility to COVID-19 Diagnosis in People with Down Syndrome Compared to the General Population: Matched-Cohort Study Using Primary Care Electronic Records in the UK. J. Gen. Intern. Med. 2022, 37, 2009–2015. [Google Scholar] [CrossRef]
- Ragnoli, B.; Pochetti, P.; Pignatti, P.; Barbieri, M.; Mondini, L.; Ruggero, L.; Trotta, L.; Montuschi, P.; Malerba, M. Sleep deprivation, immune suppression and SARS-CoV-2 infection. Int. J. Environ. Res. Public Health 2022, 19, 904. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Beller, L.; Deboutte, W.; Vieira-Silva, S.; Falony, G.; Tito, R.Y.; Rymenans, L.; Yinda, C.K.; Vanmechelen, B.; Van Espen, L.; Jansen, D. The virota and its transkingdom interactions in the healthy infant gut. Proc. Natl. Acad. Sci. USA 2022, 119, e2114619119. [Google Scholar] [CrossRef] [PubMed]
- Shamash, M.; Maurice, C.F. Phages in the infant gut: A framework for virome development during early life. ISME J. 2022, 16, 323–330. [Google Scholar] [CrossRef]
- Lee, J.E.; Walton, D.; O’Connor, C.P.; Wammes, M.; Burton, J.P.; Osuch, E.A. Drugs, guts, brains, but not rock and roll: The need to consider the role of gut microbiota in contemporary mental health and wellness of emerging adults. Int. J. Mol. Sci. 2022, 23, 6643. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G. Interactions between dietary polyphenols and aging gut microbiota: A review. BioFactors 2022, 48, 274–284. [Google Scholar] [CrossRef]
- Stefan, N.; Sippel, K.; Heni, M.; Fritsche, A.; Wagner, R.; Jakob, C.E.; Preißl, H.; Von Werder, A.; Khodamoradi, Y.; Borgmann, S. Obesity and impaired metabolic health increase risk of COVID-19-Related mortality in young and middle-aged adults to the level observed in older people: The LEOSS registry. Front. Med. 2022, 9, 1231. [Google Scholar] [CrossRef]
- Lauwers, M.; Au, M.; Yuan, S.; Wen, C. COVID-19 in joint ageing and osteoarthritis: Current status and perspectives. Int. J. Mol. Sci. 2022, 23, 720. [Google Scholar] [CrossRef] [PubMed]
- Muskiet, F.A.; Carrera-Bastos, P.; Pruimboom, L.; Lucia, A.; Furman, D. Obesity and leptin resistance in the regulation of the type I interferon early response and the increased risk for severe COVID-19. Nutrients 2022, 14, 1388. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, S.; Acuña, V.; Ceriani, R.; Cavieres, M.F.; Weinstein-Oppenheimer, C.R.; Campos-Estrada, C. Involvement of Inflammation and Its Resolution in Disease and Therapeutics. Int. J. Mol. Sci. 2022, 23, 10719. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Keshtkar, S.; Soleimanian, S.; Sabet Sarvestani, F.; Azarpira, N.; Pakbaz, S. Susceptibility to Metabolic Diseases in COVID-19: To be or Not to be an Issue. Front. Mol. Biosci. 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Marzook, H.; Ahmad, F. Comorbidities and clinical complications associated with SARS-CoV-2 infection: An overview. Clin. Exp. Med. 2022, 23, 313–331. Available online: https://pubmed.ncbi.nlm.nih.gov/35362771/ (accessed on 1 April 2022). [CrossRef] [PubMed]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The microbiota–gut–brain Axis in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Scapaticci, S.; Neri, C.; Marseglia, G.; Staiano, A.; Chiarelli, F.; Verduci, E. The impact of the COVID-19 pandemic on lifestyle behaviors in children and adolescents: An international overview. Ital. J. Pediatr. 2022, 48, 22. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. The use of antimotility drugs in COVID-19 associated diarrhea. J. Infect. 2021, 82, e19. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Bagheri, S.; Zolghadri, S.; Stanek, A. Beneficial Effects of Anti-Inflammatory Diet in Modulating Gut Microbiota and Controlling Obesity. Nutrients 2022, 14, 3985. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Goh, T.W.; Kang, M.G.; Choi, H.J.; Yeo, S.Y.; Yang, J.; Huh, C.S.; Kim, Y.Y.; Kim, Y. Perspectives and advances in probiotics and the gut microbiome in companion animals. J. Anim. Sci. Technol. 2022, 64, 197. [Google Scholar] [CrossRef]
- Włodarczyk, J.; Czerwiński, B.; Fichna, J. Short-chain fatty acids–microbiota crosstalk in the coronavirus disease (COVID-19). Pharmacol. Rep. 2022, 74, 1198–1207. [Google Scholar] [CrossRef]
- Alli, S.R.; Gorbovskaya, I.; Liu, J.C.; Kolla, N.J.; Brown, L.; Müller, D.J. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: A systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 2022, 23, 4494. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Y.; Zhao, T.; Wang, P.; Wang, Y.; Chen, Z.; Li, Y.; Xiao, D.; Fang, D. Compression experiment and numerical evaluation on mechanical responses of the lattice structures with stochastic geometric defects originated from additive-manufacturing. Compos. Part B Eng. 2020, 194, 108030. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Li, X.; Zheng, Y.; Wei, Y.; Zhang, P.; Ding, Q.; Lin, J.; Tang, S.; Zhao, Y.; et al. The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Front. Med. 2020, 14, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Sasidharan, S.P.; Yang, X. A concise review of mushrooms antiviral and immunomodulatory properties that may combat against COVID-19. Food Chem. Adv. 2022, 1, 100023. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.; Ahn, J.B.; Kim, J.H.; Ma, H.W.; Seo, D.H.; Che, X.; Park, K.C.; Jeon, J.Y.; Kim, S.Y.; et al. Lactobacillus plantarum CBT LP3 ameliorates colitis via modulating T cells in mice. Int. J. Med. Microbiol. 2020, 310, 151391. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N.H. Paneth cells, anti-microbial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Ivanov, I.I.; de Llanos Frutos, R.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.; Burwinkel, M.; Wang, Z.; Palissa, C.; Esch, B.; Twardziok, S.; Rieger, J.; Wrede, P.; Schmidt, M.F.G. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch. Virol. 2012, 158, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minato, T.; Nirasawa, S.; Sato, T.; Yamaguchi, T.; Hoshizaki, M.; Inagaki, T.; Nakahara, K.; Yoshihashi, T.; Ozawa, R.; Yokota, S.; et al. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat. Commun. 2020, 11, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumova, O.K.; Fike, A.J.; Thayer, J.L.; Nguyen, L.T.; Mell, J.C.; Pascasio, J.; Stairiker, C.; Leon, L.G.; Katsikis, P.D.; Carey, A.J. Lung transcriptional unresponsiveness and loss of early influenza virus control in infected neonates is prevented by intranasal Lactobacillus rhamnosus GG. PLoS Pathog. 2019, 15, e1008072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Anti-microbial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Mierau, I.; Kleerebezem, M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2005, 68, 705–717. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kang, J.W.; Jeon, S.-R.; Ang, L.; Lee, H.W.; Lee, M.S. Use of traditional, complementary and integrative medicine during the COVID-19 pandemic: A systematic review and meta-analysis. Front. Med. 2022, 9, 884573. [Google Scholar] [CrossRef]
- Bauer-Estrada, K.; Sandoval-Cuellar, C.; Rojas-Muñoz, Y.; Quintanilla-Carvajal, M.X. The modulatory effect of encapsulated bioactives and probiotics on gut microbiota: Improving health status through functional food. Food Funct. 2023, 14, 32–55. [Google Scholar] [CrossRef]
- Voland, L.; Le Roy, T.; Debédat, J.; Clément, K. Gut microbiota and vitamin status in persons with obesity: A key interplay. Obes. Rev. 2022, 23, e13377. [Google Scholar] [CrossRef]
- Ikhimiukor, O.O.; Odih, E.E.; Donado-Godoy, P.; Okeke, I.N. A bottom-up view of anti-microbial resistance transmission in developing countries. Nat. Microbiol. 2022, 7, 757–765. [Google Scholar] [CrossRef]
- Silva, Y.; Bernardi, A.; Frozza, R. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wu, F.-G. Probiotics armored with metal-phenolic network-based nanocoatings for gut microbiome modulation. Matter 2023, 6, 23–25. [Google Scholar] [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional foods, nutraceuticals and probiotics: A focus on human health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Küçükgöz, K.; Trząskowska, M. Nondairy probiotic products: Functional foods that require more attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Santonocito, S.; Polizzi, A.; Mauceri, R.; Troiano, G.; Lo Giudice, A.; Romano, A.; Mascitti, M.; Isola, G. A Reciprocal Link between Oral, Gut Microbiota during Periodontitis: The Potential Role of Probiotics in Reducing Dysbiosis-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 1084. [Google Scholar] [CrossRef]
- Fan, H.X.; Sheng, S.; Zhang, F. New hope for Parkinson’s disease treatment: Targeting gut microbiota. CNS Neurosci. Ther. 2022, 28, 1675–1688. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Kheirkhah, J.; Ghorbani, Z. The effects of probiotics on inflammation, endothelial dysfunction, and atherosclerosis progression: A mechanistic overview. Heart Lung Circ. 2022, 31, e45–e71. [Google Scholar] [CrossRef]
- Zanganeh, S.; Goodarzi, N.; Doroudian, M.; Movahed, E. Potential COVID-19 therapeutic approaches targeting angiotensin-converting enzyme 2; an updated review. Rev. Med. Virol. 2022, 32, e2321. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health benefits and side effects of short-chain fatty acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Kadamani, A.K.; Aly, S.; Al Sharani, S.; Liang, J.; Butcher, J.; Stintzi, A.; Matar, C.; Ismail, N. Pubertal consumption of R. badensis subspecies acadiensis modulates LPS-induced immune responses and gut microbiome dysbiosis in a sex-specific manner. Brain Behav. Immun. 2023, 107, 62–75. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Voolstra, C.R.; Sweet, M.; Duarte, C.M.; Carvalho, S.; Villela, H.; Lunshof, J.E.; Gram, L.; Woodhams, D.C.; Walter, J. Harnessing the microbiome to prevent global biodiversity loss. Nat. Microbiol. 2022, 7, 1726–1735. [Google Scholar] [CrossRef]
- Kasti, A.; Petsis, K.; Lambrinou, S.; Katsas, K.; Nikolaki, M.; Papanikolaou, I.S.; Hatziagelaki, E.; Triantafyllou, K. A combination of Mediterranean and low-FODMAP diets for managing IBS symptoms? Ask your gut! Microorganisms 2022, 10, 751. [Google Scholar] [CrossRef]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2021, 72, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, A.; Ray, M.; Ravindra, P.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef] [PubMed]
- Benarba, B.; Pandiella, A. Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Omokhua-Uyi, A.G.; Van Staden, J. Natural product remedies for COVID-19: A focus on safety. S. Afr. J. Bot. 2021, 139, 386–398. [Google Scholar] [CrossRef]
- Team, A.U.C.-R.; Akindele, A.J.; Agunbiade, F.O.; Sofidiya, M.O.; Awodele, O.; Sowemimo, A.; Ade-Ademilua, O.; Akinleye, M.O.; Ishola, I.O.; Orabueze, I. COVID-19 pandemic: A case for phytomedicines. Nat. Prod. Commun. 2020, 15, 1934578X20945086. [Google Scholar]
- Hudson, J.; Vimalanathan, S. Echinacea—A source of potent antivirals for respiratory virus infections. Pharmaceuticals 2011, 4, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabot, A.B.; Huntwork, M.P. Turmeric as a possible treatment for COVID-19-induced anosmia and ageusia. Cureus 2021, 13, e17829. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The use of antimalarial drugs against viral infection. Microorganisms 2020, 8, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [Green Version]
- Oon, S.F.; Nallappan, M.; Tee, T.T.; Shohaimi, S.; Kassim, N.K.; Sa’ariwijaya, M.S.F.; Cheah, Y.H. Xanthorrhizol: A review of its pharmacological activities and anticancer properties. Cancer Cell Int. 2015, 15, 100. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.S.; Jin, D.Q.; Mok, H.; Oh, S.J.; Lee, J.U.; Hwang, J.K.; Ha, I.; Han, J.S. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J. Neurosci. Res. 2005, 82, 831–838. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evid.-Based Complement. Altern. Med. 2020, 2020, 2560645. [Google Scholar] [CrossRef]
- Dangles, O.; Dufour, C. Flavonoid-protein interactions. In Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 443–469. [Google Scholar]
- Le Marchand, L. Cancer preventive effects of flavonoids—A review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Khan, M.; Zaeem, A.; Munir, A.; Ulfat, A.; Mumtaz, A. Plants secondary metabolites (PSMS), AS an investi-gational source against COVID-19 from flora of Pakistan. Pak. J. Bot. 2022, 54, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Plant-microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. Annu. Plant Rev. Vol. 39 Funct. Biotechnol. Plant Second. Metab. 2010, 39, 214–347. [Google Scholar]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jiang, J.; Wang, C.; Fitzgerald, M.; Hu, W.; Zhou, Y.; Zhang, H.; Chen, S. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm. Sin. B 2020, 10, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sport. Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Gonzalez, B.L.; de Oliveira, N.C.; Ritter, M.R.; Tonin, F.S.; Melo, E.B.; Sanches, A.C.C.; Fernandez-Llimos, F.; Petruco, M.V.; de Mello, J.C.P.; Chierrito, D. The naturally-derived alkaloids as a potential treatment for COVID-19: A scoping review. Phytother. Res. 2022, 36, 2686–2709. [Google Scholar] [CrossRef]
- Reider, C.A.; Chung, R.-Y.; Devarshi, P.P.; Grant, R.W.; Hazels Mitmesser, S. Inadequacy of immune health nutrients: Intakes in US adults, the 2005–2016 NHANES. Nutrients 2020, 12, 1735. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-care for common colds: The pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—Practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid.-Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced Immuno-protection against Covid-19. Ir. Med. J. 2020, 113, 58. [Google Scholar]
- Khudhair, D.H.; Al-Gareeb, A.I.; Al-Kuraishy, H.M.; El-Kadem, A.H.; Elekhnawy, E.; Negm, W.A.; Saber, S.; Cavalu, S.; Tirla, A.; Alotaibi, S.S. Combination of vitamin C and curcumin safeguards against methotrexate-induced acute liver injury in mice by synergistic antioxidant effects. Front. Med. 2022, 9, 866343. [Google Scholar] [CrossRef] [PubMed]
- Feyaerts, A.F.; Luyten, W. Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition 2020, 79, 110948. [Google Scholar] [CrossRef] [PubMed]





| Location | Microbial Diversity (Unit−1) | Representative Composition | Reference |
|---|---|---|---|
| Nasal cavity | 1 × 103 | Streptococcus * Propionibacterium Corynebacterium Moraxella | [36] |
| Nasopharynx | 1 × 103 | Streptococcus Dolosigranulum Haemophilus | |
| Oropharynx | 1 × 106 | Rothia Veillonella Leptotrichia Preuotella | |
| Lung | 1 × 102 | Veillonella Preuotella Streptococcus * Tropheryma whipplei | |
| Stomach | 1 × 101 | Lactobacillus Helicobacter Veillonella | |
| Duodenum | 1 × 103 | Streptococcus Lactococcus Staphylococcus | |
| Jejunum | 1 × 104 | Lactobacillus Streptococcus Enterococcus | |
| Ileum | 1 × 107 | Segmented filamentous bacteria Enterobacteriaceae Bacteroides Clostridium | |
| Colon | 1 × 1012 | Proteobacteria Bacteroides Clostridium Lachospiraceae Prevotellaceae |
| Category | Source | Probiotics | Mechanism and Effects | References |
|---|---|---|---|---|
| Vegetables | Sauerkraut | Lactobacillus plantarum | Promotes the growth of beneficial probiotics, boosts the immune system, ↓ stress, ↓ risk of cancer, ↓ rate of age-related loss in bone mineral density | [1,71] |
| Miso | Aspergillus oryzae | Starch hydrolysis, ↓ risk of cancer, heart disease | ||
| Kanji | Rhodotorulaglutinis | ↓ cell and tissue damage | ||
| Cassava | Galactomycesgeotrichum | Starch hydrolysis | ||
| Pickle | Pediococcus cerevisiae | ↓ chances of heart disease, stroke, cancer, and respiratory disease | ||
| Pulque | Torulasporadelbrueckii | Inhibits DPPH activity | ||
| Fruits | Masau | Saccharomyces cerevisiae | Produces folates, which ↓ the risk of Alzheimer’s disease, cardiovascular disease | |
| Cocoa | Saccharomyces cerevisiae, Halimeda opuntia | Anti-inflammatory activity, ↓ risk of irritable bowel syndrome (IBS) | ||
| Kombucha | Medusomycesgisevii lindau | Starch hydrolysis, ↓ risk of rheumatism, gout, and hemorrhoids | ||
| Olives | Candida krusei | ↑ amylase and trypsin activity | ||
| Tepache | Hanseniasporauvarum | Inhibits the growth of pathogenic bacteria | ||
| Chili Pepper | Hanseniasporaguilliermondii | Prevents colonization by pathways in the human gut | ||
| Cereals | Sourdough | Chamaerops humilis | Good hydrophobicity and hemolytic activity | |
| Ogi | Pichia kudriavzevii | Helps generate hormones and lower blood pressure | ||
| Pozol | Rhodotorulaminuta | ↓ secretion of Interleukin-8 (IL-8), ↓ cholesterol | ||
| Dairy Products | Yogurt | Lactobacillus bulgaricus, Streptococcus thermophilus | ↓ digestive problems and enhances gut microbiota | |
| Kefir | Kluyveromyces spp. | Anti-inflammatory activity and anti-aging properties | ||
| Milk | Lactobacillus fermentum | ↓ osteoporosis | ||
| Plant-Based Protein | Tempeh | Bifidobacterium | ↓ oxidative stress, ↓ cholesterol levelImproves bone health |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.; Somasundaram, I.; Das, D.; Jain Manoj, S.; Banu, H.; Mitta Suresh, P.; Paul, S.; Bisgin, A.; Zhang, H.; Sun, X.-F.; et al. Functional Foods: A Promising Strategy for Restoring Gut Microbiota Diversity Impacted by SARS-CoV-2 Variants. Nutrients 2023, 15, 2631. https://doi.org/10.3390/nu15112631
Banerjee A, Somasundaram I, Das D, Jain Manoj S, Banu H, Mitta Suresh P, Paul S, Bisgin A, Zhang H, Sun X-F, et al. Functional Foods: A Promising Strategy for Restoring Gut Microbiota Diversity Impacted by SARS-CoV-2 Variants. Nutrients. 2023; 15(11):2631. https://doi.org/10.3390/nu15112631
Chicago/Turabian StyleBanerjee, Antara, Indumathi Somasundaram, Diptimayee Das, Samatha Jain Manoj, Husaina Banu, Pavane Mitta Suresh, Sujay Paul, Atil Bisgin, Hong Zhang, Xiao-Feng Sun, and et al. 2023. "Functional Foods: A Promising Strategy for Restoring Gut Microbiota Diversity Impacted by SARS-CoV-2 Variants" Nutrients 15, no. 11: 2631. https://doi.org/10.3390/nu15112631