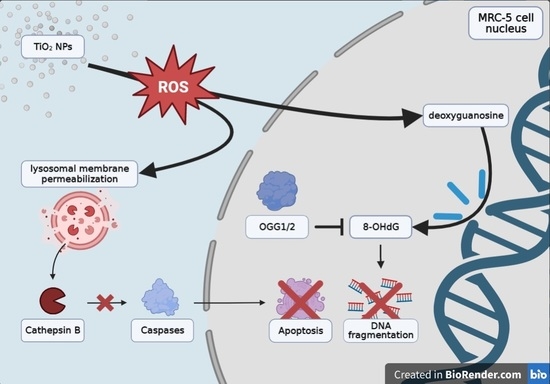
MRC-5 Human Lung Fibroblasts Alleviate the Genotoxic Effect of Fe-N Co-Doped Titanium Dioxide Nanoparticles through an OGG1/2-Dependent Reparatory Mechanism
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characteristics of TiO2 NPs
2.2. Oxidative DNA Damage Induced by TiO2 NPs in MRC-5 Cells
2.3. Influence of TiO2 NPs on the Morphology of MRC-5 Cells
2.4. Influence of TiO2 NPs on Lysosomes’ Formation and Lysosomal Membrane Integrity in MRC-5 Cells
2.5. Effect of TiO2 NPs on the Integrity of DNA from MRC-5 Cells
2.6. Cell Death Signaling in MRC-5 Cells Exposed to TiO2 NPs
2.7. The Reparatory Role of 8-oxoguanine DNA Glycosylase in MRC-5 Cells Exposed to TiO2 NPs
3. Discussion
4. Materials and Methods
4.1. Physicochemical Characterization of TiO2 NPs
4.2. Cell Culture and Treatment with TiO2 NPs
4.3. Measurement of 8-Hydroxy-2′-Deoxyguanosine Level
4.4. Fluorescence Microscopy Analysis
4.5. Comet Assay
4.6. Western Blot Analysis
4.7. Protein Concentration
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezic, I. Nanoparticles for Biomedical Application and Their Synthesis. Polymers 2022, 14, 4961. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic Nanoparticle Inks for 3D Printing of Electronics. Adv. Electron. Mater. 2019, 5, 1800831. [Google Scholar] [CrossRef]
- Jiang, C.; Cao, Y.; Xiao, G.; Zhu, R.; Lu, Y. A review on the application of inorganic nanoparticles in chemical surface coatings on metallic substrates. RSC Adv. 2017, 7, 7531–7539. [Google Scholar] [CrossRef] [Green Version]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. NaNanoparticle Res. 2012, 14, 1109. [Google Scholar] [CrossRef] [Green Version]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Nica, I.C.; Stan, M.S.; Dinischiotu, A.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Bezirtzoglou, E.; Iordache, O.G.; Varzaru, E.; et al. Innovative self-cleaning and biocompatible polyester textiles nano-decorated with Fe–N-doped titanium dioxide. Nanomaterials 2016, 6, 214. [Google Scholar] [CrossRef] [Green Version]
- Castro-Hoyos, A.M.; Manzano, M.A.R.; Maury-Ramirez, A. Challenges and opportunities of using titanium dioxide photocatalysis on cement-based materials. Coatings 2022, 12, 968. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Huerta-Garcia, E.; Perez-Arizti, J.A.; Marquez-Ramirez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; Lopez-Marure, R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Pedata, P.; Ricci, G.; Malorni, L.; Venezia, A.; Cammarota, M.; Volpe, M.G.; Iannaccone, N.; Guida, V.; Schiraldi, C.; Romano, M.; et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res. Int. 2019, 119, 634–642. [Google Scholar] [CrossRef]
- Ling, C.; An, H.; Li, L.; Wang, J.; Lu, T.; Wang, H.; Hu, Y.; Song, G.; Liu, S. Genotoxicity evaluation of titanium dioxide nanoparticles in vitro: A systematic review of the literature and meta-analysis. Biol. Trace Elem. Res. 2020, 199, 2057–2076. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination temperature effect on titanium oxide (TiO2) nanoparticles synthesis. Optik 2021, 241, 166934. [Google Scholar] [CrossRef]
- Dorosheva, I.B.; Valeeva, A.A.; Rempel, A.A.; Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N. Synthesis and Physicochemical Properties of Nanostructured TiO2 with Enhanced Photocatalytic Activity. Inorg. Mater. 2021, 57, 503–510. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef] [Green Version]
- Manuputty, M.Y.; Xu, R.; Kraft, M. Effects of particle collection in a premixed stagnation flame synthesis of sub-stoichiometric TiO2-x nanoparticles. Chem. Eng. Sci. 2023, 265, 118155. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef]
- Numano, T.; Xu, J.; Futakuchi, M.; Fukamachi, K.; Alexander, D.B.; Furukawa, F.; Kanno, J.; Hirose, A.; Tsuda, H.; Suzui, M. Comparative Study of Toxic Effects of Anatase and Rutile Type Nanosized Titanium Dioxide Particles in vivo and in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iswarya, V.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. Individual and binary toxicity of anatase and rutile nanoparticles towards Ceriodaphnia dubia. Aquat. Toxicol. 2016, 178, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Prokopiuk, V.; Yefimova, S.; Onishchenko, A.; Kapustnik, V.; Myasoedov, V.; Maksimchuk, P.; Butov, D.; Bespalova, I.; Tkachenko, A. Assessing the Cytotoxicity of TiO2−x Nanoparticles with a Different Ti3+(Ti2+)/Ti4+ Ratio. Biol. Trace Elem. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Xiong, S.; George, S.; Ji, Z.; Lin, S.; Yu, H.; Damoiseaux, R.; France, B.; Ng, K.W.; Loo, S.C.J. Size of TiO2 nanoparticles influences their phototoxicity: An in vitro investigation. Arch. Toxicol. 2013, 87, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Yin, L.; Pu, Y.; Liang, G.; Zhang, J.; Su, Y.; Xiao, Z.; Ye, B. Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intra-tracheal instillation in rats. Prog. Nat. Sci. 2009, 19, 573–579. [Google Scholar] [CrossRef]
- Hussain, S.; Boland, S.; Baeza-Squiban, A.; Hamel, R.; Thomassen, L.C.J.; Martens, J.A.; Billon-Galland, M.A.; Fleury-Feith, J.; Moisan, F.; Pairon, J.C.; et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: Role of particle surface area and internalized amount. Toxicology 2009, 260, 142–149. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brassinne, F.; Sebaihi, N.; Petry, J.; Cokic, S.M.; van Landuyt, K.L.; Godderis, L.; Mast, J.; Lison, D.; Hoet, P.H.; et al. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part. Fibre Toxicol. 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhou, T.; Yang, W.; Liu, J.; Shao, L. Contribution of oxidative stress to TiO2 nanoparticle-induced toxicity. Environ. Toxicol. Pharmacol. 2016, 48, 130–140. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [Green Version]
- Beberok, A.; Wrześniok, D.; Szlachta, M.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Lomefloxacin Induces Oxidative Stress and Apoptosis in COLO829 Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 2194. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Liu, A.; Anadon, A.; Rodriguez, J.L.; Martinez-Larranaga, M.R.; Yuan, Z.; Martinez, M.A. Paracetamol: Overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab. Rev. 2017, 49, 395–437. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wołejko, E.; Wydro, U.; Butarewicz, A. The impact of pesticides on oxidative stress level in human organism and their activity as an endocrine disruptor. J. Environ. Sci. Health B 2017, 52, 483–494. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Chaudhary, S.; Kim, M.H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Kamiya, H. Mutations induced by 8-hydroxyguanine (8-oxo-7,8-dihydroguanine), a representative oxidized base, in mammalian cells. Genes Environ. 2017, 39, 2. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and the Council as Regards the Food Additive Titanium Dioxide (E171); Official Journal of the European Union, L11/1; The European Union: Brussels, Belgium, 2022.
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne de Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol. Physiol. 2009, 22, 266–275. [Google Scholar] [CrossRef]
- Vujovic, M.; Kostic, E. Titanium dioxide and zinc oxide nanoparticles in sunscreens: A review of toxicological data. J. Cosmet. Sci. 2019, 70, 223–234. [Google Scholar]
- IARC (International Agency for Research on Cancer). Carbon Black, Titanium Dioxide, and Talc. Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93, pp. 1–452. [Google Scholar]
- Commission Regulation (EU) 2019/1857 of 6 November 2019 Amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; Official Journal of the European Union, L286/3; The European Union: Brussels, Belgium, 2019.
- Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 Amending, for the Purposes of Its Adaptation to Technical and Scientific Progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures and Correcting that Regulation; Official Journal of the European Union, L44/1; The European Union: Brussels, Belgium, 2020.
- NIOSH (National Institute for Occupational Safety and Health). Occupational exposure to titanium dioxide. Curr. Intell. Bull. 2011, 63, 120. [Google Scholar]
- ANSES (Agence Nationale de Securité Sanitaire de l’Alimentation, de l’Environnement et du Travail). Valeurs limites d’exposition en milieu professionnel. Le dioxyde de titane sous forme nanometrique (TiO2-NP, P25). Rapport d’expertise collective. 2020. Available online: https://www.anses.fr/fr/system/files/VSR2019SA0109Ra.pdf (accessed on 17 January 2023).
- Belade, E.; Armand, L.; Martinon, L.; Kheuang, L.; Fleury-Feith, J.; Baeza-Squiban, A.; Lanone, S.; Billon-Galland, M.A.; Pairon, J.C.; Boczkowski, J. A comparative transmission electron microscopy study of titanium dioxide and carbon black nanoparticles uptake in human lung epithelial and fibroblast cell lines. Toxicol. Vitr. 2012, 26, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Janer, G.; del Molino, E.M.; Fernandez-Rosas, E.; Fernandez, A.; Vazquez-Campos, S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef]
- Huerta-Garcia, E.; Marquez-Ramirez, S.G.; Ramos-Godinez, M.P.; Lopez-Saavedra, A.; Herrera, L.A.; Parra, A.; Alfaro-Moreno, E.; Gomez, E.O.; Lopez-Marure, R. Internalization of titanium dioxide nanoparticles by glial cells is given at short times and is mainly mediated by actin reorganization-dependent endocytosis. NeuroToxicology 2015, 51, 27–37. [Google Scholar] [CrossRef]
- Jugan, M.L.; Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 2012, 6, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Vignardi, C.P.; Hasue, F.M.; Sartorio, P.V.; Cardoso, C.M.; Machado, A.S.D.; Passos, M.J.A.C.R.; Santos, T.C.A.; Nucci, J.M.; Hewer, T.L.R.; Watanabe, I.S.; et al. Genotoxicity, potential cytotoxicity and cell uptake of titanium dioxide nanoparticles in the marine fish Trachinotus carolinus. Aquat. Toxicol. 2015, 158, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Mackevica, A.; Johansson, B.R.; Sturve, J. Endocytosis, intracellular fate, accumulation, and agglomeration of titanium dioxide (TiO2) nanoparticles in the rainbow trout liver cell line RTL-W1. Environ. Sci. Pollut. Res. 2019, 26, 15354–15372. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.R.; Gemini-Piperni, S.; Travassos, R.; Lemgruber, L.; Silva, R.C.; Rossi, A.L.; Farina, M.; Anselme, K.; Shokuhfar, T.; Shahbazian-Yassar, R.; et al. Trojan-Like Internalization of Anatase Titanium Dioxide Nanoparticles by Human Osteoblast Cells. Sci. Rep. 2016, 6, 23615. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Friehs, G.; Froelich, K.; Ginzkey, C.; Koehler, C.; Scherzed, A.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Intracellular distribution, geno- and cytotoxic effects of nanosized titanium dioxide particles in the anatase crystal phase on human nasal mucosa cells. Toxicol. Lett. 2010, 195, 9–14. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Ghiazza, M.; Alloa, E.; Oliaro-Bosso, S.; Viola, F.; Livraghi, S.; Rembges, D.; Capomaccio, R.; Rossi, F.; Ponti, J.; Fenoglio, I. Inhibition of the ROS-mediated cytotoxicity and genotoxicity of nano-TiO2 toward human keratinocyte cells by iron doping. J. Nanoparticle Res. 2014, 16, 2263. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Nica, I.C.; Stan, M.S.; Dinischiotu, A. Current photocatalytic applications of nano-scaled titanium dioxide in the new era of ”smart” technologies. Rev. Biol. Biomed. Sci. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells. Sci. Rep. 2016, 6, 30196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, J.; Siddiqui, M.A.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Khan, S.T.; Wahab, R.; Al-Khedhairy, A.A.; Al-Salim, A.; Musarrat, J.; et al. Copper doping enhanced the oxidative stress-mediated cytotoxicity of TiO2 nanoparticles in A549 cells. Hum. Exp. Toxicol. 2018, 37, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Diamandescu, L.; Feder, M.; Vasiliu, F.; Tanase, L.; Sobetkii, A.; Dumitrescu, I.; Teodorescu, M.; Popescu, T. Hydrothermal route to (Fe, N) codoped titania photocatalysts with increased visible light activity. Ind. Textila 2017, 68, 303–308. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Siwach, R.; Kumar, S.; Alhazaa, A.N. Role of Fe doping in tuning photocatalytic and photoelectrochemical properties of TiO2 for photodegradation of methylene blue. Opt. Laser Technol. 2019, 118, 170–178. [Google Scholar] [CrossRef]
- Matias, L.M.; Pimentel, A.; Reis-Machado, A.S.; Rodrigues, J.; Deuermeier, J.; Fortunato, E.; Martins, R.; Nunes, D. Enhanced Fe-TiO2 Solar Photocatalysts on Porous Platforms for Water Purification. Nanomaterials 2022, 12, 1005. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Ji, Z.; Henderson, B.L.; Xia, T.; Li, L.J.; Zink, J.I.; Nel, A.E.; Madler, L. Role of Fe doping in tuning the band gap of TiO2 for photo-oxidation induced cytotoxicity paradigm. J. Am. Chem. Soc. 2011, 133, 11270–11278. [Google Scholar] [CrossRef] [Green Version]
- Nica, I.C.; Stan, M.S.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Dumitrescu, I.; Ignat, M.; Feder, M.; Tanase, L.C.; et al. Interaction of new-developed TiO2-based photocatalytic nanoparticles with pathogenic microorganisms and human dermal and pulmonary fibroblasts. Int. J. Mol. Sci. 2017, 18, 249. [Google Scholar] [CrossRef] [Green Version]
- Nica, I.C.; Miu, B.A.; Stan, M.S.; Diamandescu, L.; Dinischiotu, A. Could Iron-Nitrogen Doping Modulate the Cytotoxicity of TiO2 Nanoparticles? Nanomaterials 2022, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Iwakuma, T. Non-Canonical Cell Death Induced by p53. Int. J. Mol. Sci. 2016, 17, 2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Castro, M.; Bunt, G.; Wouters, F. Cathepsin B launches an apoptotic exit effort upon cell death-associated disruption of lysosomes. Cell Death Discov. 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada-Oikawa, S.; Ichihara, G.; Fukatsu, H.; Shimanuki, Y.; Tanaka, N.; Watanabe, E.; Suzuki, Y.; Murakami, M.; Izuoka, K.; Chang, J.; et al. Titanium Dioxide Particle Type and Concentration Influence the Inflammatory Response in Caco-2 Cells. Int. J. Mol. Sci. 2016, 17, 576. [Google Scholar] [CrossRef] [Green Version]
- Dorier, M.; Beal, D.; Tisseyre, C.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Rabilloud, T.; Carrière, M. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ. Sci. Nano 2019, 6, 1549–1561. [Google Scholar] [CrossRef] [Green Version]
- Brandão, F.; Fernández-Bertólez, N.; Rosário, F.; Bessa, M.J.; Fraga, S.; Pásaro, E.; Teixeira, J.P.; Laffon, B.; Valdiglesias, V.; Costa, C. Genotoxicity of TiO2 Nanoparticles in Four Different Human Cell Lines (A549, HEPG2, A172 and SH-SY5Y). Nanomaterials 2020, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Lojk, J.; Repas, J.; Veranic, P.; Bregar, V.B.; Pavlin, M. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicology 2020, 432, 152364. [Google Scholar] [CrossRef]
- Mao, Z.; Xu, B.; Ji, X.; Zhou, K.; Zhang, X.; Chen, M.; Han, X.; Tang, Q.; Wang, X.; Xia, Y. Titanium dioxide nanoparticles alter cellular morphology via disturbing the microtubule dynamics. Nanoscale 2015, 7, 8466. [Google Scholar] [CrossRef]
- Ibrahim, M.; Schoelermann, J.; Mustafa, K.; Cimpan, M.R. TiO2 nanoparticles disrupt cell adhesion and the architecture of cytoskeletal networks of human osteoblast-like cells in a size dependent manner. J. Biomed. Mater. Res. Part A 2018, 106, 2582–2593. [Google Scholar] [CrossRef]
- Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology 2012, 302, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Alcaraz, A.; Delgado-Buenrostro, N.L.; Ispanixtlahuatl-Meráz, O.; Freyre-Fonseca, V.; Flores-Flores, J.O.; Ganem-Rondero, A.; Vaca-Paniagua, F.; Ramos-Godinez, M.P.; Morales-Barcenas, R.; Sánchez-Pérez, Y.; et al. Irreversible disruption of the cytoskeleton as induced by non-cytotoxic exposure to titanium dioxide nanoparticles in lung epithelial cells. Chem. Biol. Interact. 2020, 323, 109063. [Google Scholar] [CrossRef]
- Akamatsu, M.; Vasan, R.; Serwas, D.; Ferrin, M.A.; Rangamani, P.; Drubin, D.G. Principles of self-organization and load adaptation by the actin cytoskeleton during clathrin-mediated endocytosis. eLife 2020, 9, e49840. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Lamaze, C. Clathrin-Dependent or Not: Is It Still the Question? Traffic 2002, 3, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.T.; Arora, H.; Paunesku, T.; Wu, A.; Brown, E.M.B.; Doty, C.; Kremer, J.; Woloschak, G. Endocytosis of titanium dioxide nanoparticles in prostate cancer PC-3M cells. Nanomedicine 2011, 7, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Santiso, R.; Tamayo, M.; Gosalvez, J.; Meseguer, M.; Garrido, N.; Fernandez, J.L. Simultaneous determination in situ of DNA fragmentation and 8-oxoguanine in human sperm. Fertil. Steril. 2010, 93, 314–318. [Google Scholar] [CrossRef]
- Yu, T.; Slone, J.; Liu, W.; Barnes, R.; Opresko, P.L.; Wark, L.; Mai, S.; Horvath, S.; Huang, T. Premature aging is associated with higher levels of 8-oxoguanine and increased DNA damage in the Polg mutator mouse. Aging Cell 2022, 21, e13669. [Google Scholar] [CrossRef]
- Hackenberg, S.; Friehs, G.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Scherzed, A.; Burghartz, M.; Kleinsasser, N. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ. Mol. Mutagen. 2011, 52, 264–268. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.F.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Liao, F.; Chen, L.; Liu, Y.; Zhao, D.; Peng, W.; Wang, W.; Feng, S. The size-dependent genotoxic potentials of titanium dioxide nanoparticles to endothelial cells. Environ. Toxicol. 2019, 34, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver Nanoparticle-Induced Autophagic Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gomez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- D’Augustin, O.; Huet, S.; Campalans, A.; Radicella, J.P. Lost in the Crowd: How Does Human 8-Oxoguanine DNA Glycosylase 1 (OGG1) Find 8-Oxoguanine in the Genome? Int. J. Mol. Sci. 2020, 21, 8360. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhu, X.; Fan, C.; Xu, S.; Wang, Y.; Zhou, Y. Oxidative damage and OGG1 expression induced by a combined effect of titanium dioxide nanoparticles and lead acetate in human hepatocytes. Environ. Toxicol. 2012, 27, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Zijno, A.; De Angelis, I.; De Berardis, B.; Andreoli, C.; Russo, M.T.; Pietraforte, D.; Scorza, G.; Degan, P.; Ponti, J.; Rossi, F.; et al. Different mechanisms are involved in oxidative DNA damage and genotoxicity induction by ZnO and TiO2 nanoparticles in human colon carcinoma cells. Toxicol. Vitr. 2015, 29, 1503–1512. [Google Scholar] [CrossRef]
- Xia, B.; Chen, J.; Zhou, Y. Cellular Oxidative Damage of HEK293T Cells Induced by Combination of CdCl2 and Nano-TiO2. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 290–294. [Google Scholar] [CrossRef]
- Offer, H.; Zurer, I.; Banfalvi, G.; Reha’k, M.; Falcovitz, A.; Milyavsky, M.; Goldfinger, N.; Rotter, V. p53 Modulates Base Excision Repair Activity in a Cell Cycle-specific Manner after Genotoxic Stress. Cancer Res. 2001, 61, 88–96. [Google Scholar] [PubMed]
- Sobol, R.W.; Kartalou, M.; Almeida, K.H.; Joyce, D.F.; Engelward, B.P.; Horton, J.K.; Prasad, R.; Samson, L.D.; Wilson, S.H. Base Excision Repair Intermediates Induce p53-independent Cytotoxic and Genotoxic Responses. J. Biol. Chem. 2003, 278, 39951–39959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]








| Sample | Ti 2p3/2 | O 1s | Fe 2p3/2 | N 1s |
|---|---|---|---|---|
| Binding Energy (eV) | ||||
| TiO2 P25 | 458.65 459.63 | 529.98 | ||
| 531.03 | - | - | ||
| 532.16 | ||||
| TiO2 P25-Fe(1%)-N | 458.08 | 529.42 | 399.62 401.19 | |
| 459.30 | 530.75 | 710.40 | ||
| 460.27 | 531.81 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miu, B.A.; Voinea, I.C.; Diamandescu, L.; Dinischiotu, A. MRC-5 Human Lung Fibroblasts Alleviate the Genotoxic Effect of Fe-N Co-Doped Titanium Dioxide Nanoparticles through an OGG1/2-Dependent Reparatory Mechanism. Int. J. Mol. Sci. 2023, 24, 6401. https://doi.org/10.3390/ijms24076401
Miu BA, Voinea IC, Diamandescu L, Dinischiotu A. MRC-5 Human Lung Fibroblasts Alleviate the Genotoxic Effect of Fe-N Co-Doped Titanium Dioxide Nanoparticles through an OGG1/2-Dependent Reparatory Mechanism. International Journal of Molecular Sciences. 2023; 24(7):6401. https://doi.org/10.3390/ijms24076401
Chicago/Turabian StyleMiu, Bogdan Andrei, Ionela Cristina Voinea, Lucian Diamandescu, and Anca Dinischiotu. 2023. "MRC-5 Human Lung Fibroblasts Alleviate the Genotoxic Effect of Fe-N Co-Doped Titanium Dioxide Nanoparticles through an OGG1/2-Dependent Reparatory Mechanism" International Journal of Molecular Sciences 24, no. 7: 6401. https://doi.org/10.3390/ijms24076401