Current Status of Research on Gut Metabolites and Microbiota
A topical collection in Pathogens (ISSN 2076-0817).
Viewed by 37604Editor
Interests: microbial ecology; infectious diseases; human microbiome; microbial interactions; antibiotic resistance; omics
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
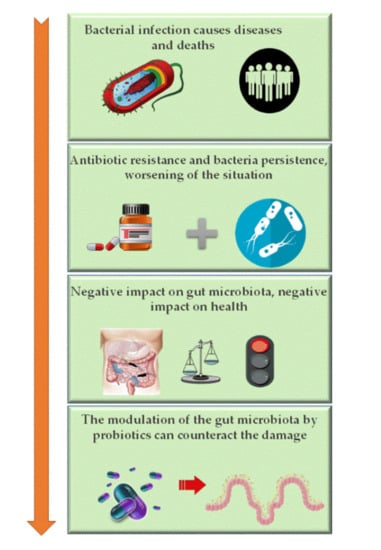
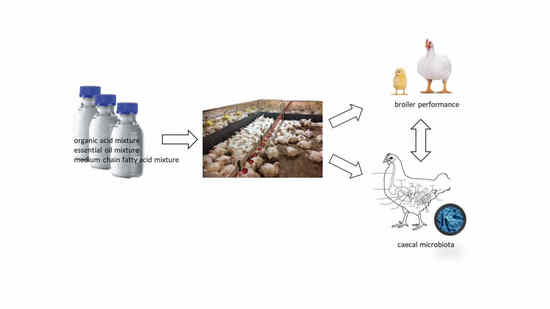
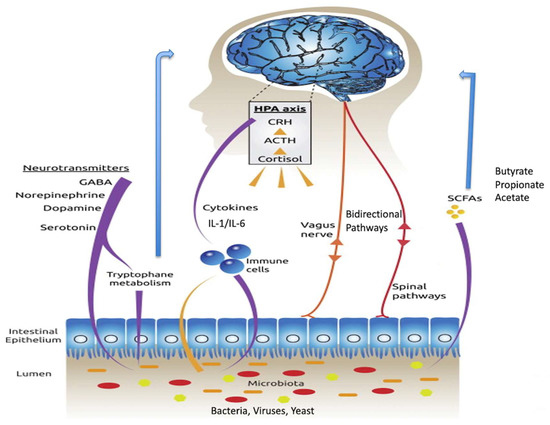
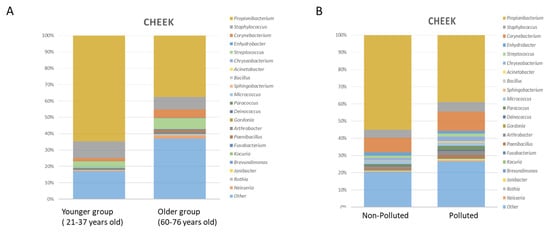
Metabolites are small molecules that derive from cell metabolism. In the last few years, the role of metabolites produced in the gut by the microbiome–host system has attracted much attention. They can be considered to be “molecular words” in the communication language within the gut microbial community (microbe–microbe) and other microbial communities in the body, such as those inhabiting the skin, the oral cavity, or the respiratory tract, as well as to and from the host (microbe–host). Metabolites circulating in the gut are involved in several host physiological processes, such as nutrition, immune system stimulation, epithelium maintenance, and colonization resistance. Interruption of the metabolite-based dialogue between the microbiota and the host leads to disease, not only intestinal but affecting other organs or systemic. It is the case for intestinal inflammatory bowel disease, Clostridium difficile colitis, colorectal cancer, asthma, and some metabolic, neurological, reproductive, and immune-system-associated disorders where the gut metabolome (the complete set of small molecules from the gut) plays a part in their outcome. Omics approaches, especially metagenomics combined with metabolomics, have significantly contributed to the study of the function of critical metabolites for health, such as short-chain fatty acids, bile acids, amino acids, and polyamines and to the identification of their producers. However, our knowledge of the ecological web of metabolic interactions is filled with gaps due to the ecological complexity of the gut microbiome–host interplay. The area of microbiome metabolomics is promising and already expanding, especially regarding its therapeutic applications. We thus invite contributions to this Topic Collection in the form of original research articles, case reports, short communications, or review papers that cover any aspect of gut metabolites and the microbiome, and host–microbiome interplay, including new research, new data, novel methodologies, and future perspectives.
Dr. Ana Elena Pérez Cobas
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pathogens is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- gut
- microbiota
- metabolites
- metabolomics
- interaction
- physiology