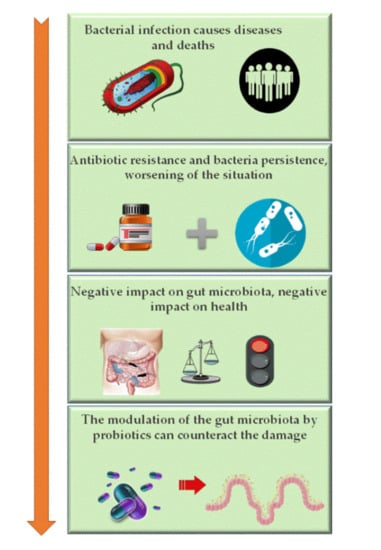
Effect of Probiotics on Host-Microbiota in Bacterial Infections
Abstract
:1. Introduction
1.1. Gut Microbiota and Infection-Related Dysbiosis
1.2. Pathogenic Bacteria and Antibiotics as Disruptors of Microbiota
2. Use of Probiotics and Their Impact on Microbiota in Infection Diseases
3. Probiotics as Gut Microbiota Modulators to Counteract the Bacterial Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Baron, S.A.; Diene, S.M.; Rolain, J.-M. Human microbiomes and antibiotic resistance. Hum. Microbiome J. 2018, 10, 43–52. [Google Scholar] [CrossRef]
- Pham, T.A.N.; Lawley, T.D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr. Opin. Microbiol. 2014, 17, 67–74. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Stecher, B.; Robbiani, R.; Walker, A.W.; Westendorf, A.M.; Barthel, M.; Kremer, M.; Chaffron, S.; Macpherson, A.J.; Buer, J.; Parkhill, J.; et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007, 5, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.J.; Bates, K.A.; King, K.C. Host microbiota can facilitate pathogen infection. PLoS Pathog 2021, 17, e1009514. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Johnson, J.R.; Clabots, C.; Porter, S.B.; Bender, T.; Johnston, B.D.; Thuras, P. Intestinal Persistence of Colonizing Escherichia coli Strains, Especially ST131-H30, in Relation to Bacterial and Host Factors. J. Infect. Dis. 2022, 225, 2197–2207. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Persistence of Intracellular Bacterial Pathogens—With a Focus on the Metabolic Perspective. Front. Cell. Infect. Microbiol. 2021, 10, 615450. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.W.; Saunders, M.E.; Jett, B.D. (Eds.) Chapter 13—Immunity to Infection. In Primer to the Immune Response, 2nd ed.; Academic Cell: Boston, MA, USA, 2014; pp. 295–332. [Google Scholar]
- Fauvart, M.; De Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.Y.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Forssten, S.; Hibberd, A.A.; Lyra, A.; Stahl, B. Probiotic approach to prevent antibiotic resistance. Ann. Med. 2016, 48, 246–255. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Li, M.; Wang, B.; Zhang, M.; Rantalainen, M.; Wang, S.; Zhou, H.; Zhang, Y.; Shen, J.; Pang, X.; Zhang, M.; et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. USA 2008, 105, 2117–2122. [Google Scholar] [CrossRef]
- Reese, A.T.; Cho, E.H.; Klitzman, B.; Nichols, S.P.; Wisniewski, N.A.; Villa, M.M.; Durand, H.K.; Jiang, S.; Midani, F.S.; Nimmagadda, S.N.; et al. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. Elife 2018, 19, e35987. [Google Scholar] [CrossRef]
- Kaur, H.; Ali, S.A. Probiotics and gut microbiota: Mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar] [CrossRef]
- Knipe, H.; Temperton, B.; Lange, A.; Bass, D.; Tyler, C.R. Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Rev. Aquac. 2021, 13, 324–352. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.M.; Dolovich, L.R.; Lee, C.H. Prevention of Clostridium difficile infection with Saccharomyces boulardii: A systematic review. Can. J. Gastroenterol. 2009, 23, 817–821. [Google Scholar] [CrossRef]
- Batra, P.; Soni, K.D.; Mathur, P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: An updated systematic review and meta-analysis of randomized control trials. J. Intensive Care 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, B.; Mangell, P.; Thorlacius, H. Use of Probiotics as Prophylaxis for Postoperative Infections. Nutrients 2011, 3, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Eurosurveillance Editorial Team. WHO member states adopt global action plan on antimicrobial resistance. Euro Surveill. 2015, 20, 21137. [Google Scholar]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methé, B.A.; Huttenhower, C. The Human Microbiome Project: A community resource for the healthy human microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef]
- Montassier, E.; Valdés-Mas, R.; Batard, E.; Zmora, N.; Dori-Bachash, M.; Suez, J.; Elinav, E. Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner. Nat. Microbiol. 2021, 6, 1043–1054. [Google Scholar] [CrossRef]
- Sherman, P.M.; Johnson-Henry, K.C.; Yeung, H.P.; Ngo, P.S.C.; Goulet, J.; Tompkins, T.A. Probiotics Reduce Enterohemorrhagic Escherichia coli O157:H7- and Enteropathogenic E. coli O127:H6-Induced Changes in Polarized T84 Epithelial Cell Monolayers by Reducing Bacterial Adhesion and Cytoskeletal Rearrangements. Infect. Immun. 2005, 73, 5183–5188. [Google Scholar] [CrossRef]
- Carter, K.A.; Srinivasan, S.; Fiedler, T.L.; Anzala, O.; Kimani, J.; Mochache, V.; Wallis, J.M.; Fredricks, D.N.; McClelland, R.S.; Balkus, J.E. Vaginal Bacteria and Risk of Incident and Persistent Infection with High-Risk Subtypes of Human Papillomavirus: A Cohort Study Among Kenyan Women. Sex. Transm. Dis. 2021, 48, 499–507. [Google Scholar] [CrossRef]
- Ratten, L.; Plummer, E.; Murray, G.; Danielewski, J.; Fairley, C.; Garland, S.; Hocking, J.; Tachedjian, G.; Chow, E.; Bradshaw, C.; et al. Sex is associated with the persistence of non-optimal vaginal microbiota following treatment for bacterial vaginosis: A prospective cohort study. Br. J. Obstet. Gynaecol. 2021, 128, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Nakagawa, M.; Coleman, H.N.; Owens, S.M.; Greenfield, W.W.; Sasagawa, T.; Robeson, M.S., 2nd. Evaluation of DNA extraction protocols from liquid-based cytology specimens for studying cervical microbiota. PLoS ONE 2021, 16, e0237556. [Google Scholar] [CrossRef] [PubMed]
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D.; et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, J.; Lu, Y.; Cai, Q.; Liu, H.; Xu, C. Cervical microbiome is altered in cervical intraepithelial neoplasia after loop electrosurgical excision procedure in china. Sci. Rep. 2018, 8, 4923. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Wu, J.; Wu, Y.; Peng, W.; Li, H.; Wang, J.; Tang, X.; Peng, Y.; Fu, X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 2018, 67, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M.; Blaser, M.J.; Britton, R.; Walter, J. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Hod, K.; Dekel, R.; Aviv Cohen, N.; Sperber, A.; Ron, Y.; Boaz, M.; Berliner, S.; Maharshak, N. The effect of a multispecies probiotic on microbiota composition in a clinical trial of patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13456. [Google Scholar] [CrossRef]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Taverniti, V.; Koirala, R.; Dalla Via, A.; Gargari, G.; Leonardis, E.; Arioli, S.; Guglielmetti, S. Effect of Cell Concentration on the Persistence in the Human Intestine of Four Probiotic Strains Administered through a Multispecies Formulation. Nutrients 2019, 11, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, A.; Fatani, A.; Ford, A.L.; Piano, A.; Nagulesapillai, V.; Auger, J.; MacPherson, C.W.; Christman, M.C.; Tompkins, T.A.; Dahl, W.J. Safety and Effect of a Low- and High-Dose Multi-Strain Probiotic Supplement on Microbiota in a General Adult Population: A Randomized, Double-Blind, Placebo-Controlled Study. J. Diet. Suppl. 2021, 18, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Bordalo Tonucci, L.; Dos Santos, K.M.O.; De Luces Fortes Ferreira, C.L.; Ribeiro, S.M.R.; De Oliveira, L.L.; Martino, H.S.D. Gut microbiota and probiotics: Focus on diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2017, 57, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Kurtz, C.B.; Millet, Y.A.; Puurunen, M.K.; Perreault, M.; Charbonneau, M.R.; Isabella, V.M.; Kotula, J.W.; Antipov, E.; Dagon, Y.; Denney, W.S.; et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 2019, 11, eaau7975. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J. Intelligent Algorithm-Based Gastrointestinal X-ray Examination in Evaluating the Therapeutic Effect of Probiotics Combined with Triple Therapy on Children with Helicobacter Infection. Contrast Media Mol. Imaging 2022, 2022, 8464361. [Google Scholar] [CrossRef]
- Rauseo, A.M.; Hink, T.; Reske, K.A.; Seiler, S.M.; Bommarito, K.M.; Fraser, V.J.; Burnham, C.D.; Dubberke, E.R. A randomized controlled trial of Lactobacillus rhamnosus GG on antimicrobial-resistant organism colonization. Infect. Control Hosp. Epidemiol. 2022, 43, 167–173. [Google Scholar] [CrossRef]
- Viazis, N.; Argyriou, K.; Kotzampassi, K.; Christodoulou, D.K.; Apostolopoulos, P.; Georgopoulos, S.D.; Liatsos, C.; Giouleme, O.; Koustenis, K.; Veretanos, C.; et al. A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates. Nutrients 2022, 14, 632. [Google Scholar] [CrossRef]
- Singh, G.; Haileselassie, Y.; Briscoe, L.; Bai, L.; Patel, A.; Sanjines, E.; Hendler, S.; Singh, P.K.; Garud, N.R.; Limketkai, B.N.; et al. The effect of gastric acid suppression on probiotic colonization in a double blinded randomized clinical trial. Clin. Nutr. ESPEN 2022, 47, 70–77. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, C.C.; Huang, Y.C.; Tseng, C.C.; Hsu, J.T.; Lin, Y.F.; Fang, Y.J.; Wu, M.S.; Liou, J.M. The efficacy of Lactobacillus acidophilus and rhamnosus in the reduction of bacterial load of Helicobacter pylori and modification of gut microbiota-a double-blind, placebo-controlled, randomized trial. Helicobacter 2021, 26, e12857. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Verbelen, V.; Van Den Driessche, M.; Melnik, E.; Vanheule, G.; Marot, J.C.; Cani, P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota with Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. Public Health 2020, 8, 578089. [Google Scholar] [CrossRef] [PubMed]
- Guillemard, E.; Poirel, M.; Schäfer, F.; Quinquis, L.; Rossoni, C.; Keicher, C.; Wagner, F.; Szajewska, H.; Barbut, F.; Derrien, M.; et al. A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy. Nutrients 2021, 13, 3171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xiao, S.; Li, S.; Suo, B.; Wang, Y.; Meng, L.; Liu, Z.; Yin, Z.; Xue, Y.; Zhou, L. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter 2021, 26, e12848. [Google Scholar] [CrossRef]
- Dall, L.B.; Lausch, K.R.; Gedebjerg, A.; Fuursted, K.; Storgaard, M.; Larsen, C.S. Do probiotics prevent colonization with multi-resistant Enterobacteriaceae during travel? A randomized controlled trial. Travel Med. Infect. Dis. 2019, 27, 81–86. [Google Scholar] [CrossRef]
- Li, K.L.; Wang, B.Z.; Li, Z.P.; Li, Y.L.; Liang, J.J. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J. Pediatr. WJP 2019, 15, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, X.G.; Wang, J.; Chen, Y.J.; Qin, H.L.; Yang, R. Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure: An open-label single-center randomized parallel controlled study. World J. Pediatr. WJP 2021, 17, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Paitán, E.; Sotelo, A.; Zúñiga, D.; Vílchez, C. Molecular characterization of bacteria with probiotic potential isolated from stool of human neonates. Rev. Peru. De Biol. 2019, 26, 119–130. [Google Scholar] [CrossRef]
- Brandon, A.M.; Garcia, A.M.; Khlystov, N.A.; Wu, W.-M.; Criddle, C.S. Enhanced Bioavailability and Microbial Biodegradation of Polystyrene in an Enrichment Derived from the Gut Microbiome of Tenebrio molitor (Mealworm Larvae). Environ. Sci. Technol. 2021, 55, 2027–2036. [Google Scholar] [CrossRef]
- Aggarwal, N.; Breedon, A.M.E.; Davis, C.M.; Hwang, I.Y.; Chang, M.W. Engineering probiotics for therapeutic applications: Recent examples and translational outlook. Curr. Opin. Biotechnol. 2020, 65, 171–179. [Google Scholar] [CrossRef]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan Khatibi, S.M.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The Battle of Probiotics and Their Derivatives Against Biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2020, 9, 27. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell Infect. Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, I.; Cohen-Cymberknoh, M.; Zamir, G.; Tsesis, I.; Rosen, E. Targeting Persistent Biofilm Infections: Reconsidering the Topography of the Infection Site during Model Selection. Microorganisms 2022, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 2020, 77, 1319–1343. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Liu, J.; Liu, C.; Qiao, J. Vertical and horizontal quorum-sensing-based multicellular communications. Trends Microbiol. 2021, 29, 1130–1142. [Google Scholar] [CrossRef]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- de Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]

| Study Design | Study Population | Aim/Intervention | Main Effects on Microbiota | Reference |
|---|---|---|---|---|
| Clinical trial | N = 40 18–60 y/o | To evaluate the persistence in the human GI tract of a probiotic mix (Bifidobacterium animalis subsp. lactis Bl-04, Lactobacillus acidophilus La-14, Lactobacillus plantarum SDZ-11, and Lactobacillus paracasei SDZ-22) supplement | Higher doses of probiotics: ↑ recovery in the feces of healthy adults | [42] |
| Clinical trial | N = 20 28–45 y/o | To evaluate the effect of Bifidobacterium longum BB536 and L. rhamnosus HN001 on the intestinal environment | Probiotics modulated gut microbiota ↓ damage by harmful bacteria | [44] |
| Randomized controlled trial | 18–34 y/o | Dose-response analysis of probiotics (containing Lactobacillus helveticus R0052, Lactobacillus rhamnosus R0011, Lactobacillus casei R0215, Pediococcus acidilactici R1001, Bifidobacterium breve R0070, Bifidobacterium longum ssp. longum BB536, Lactobacillus plantarum R1012, and Lactococcus lactis ssp. lactis R1058) supplementation to evaluate microbiota composition, transit persistence, and safety in adults | ↑ Bacteriodales ↓ Holdemania | [43] |
| Randomized double-blinded | N = 52 18–64 y/o | To evaluate the decrease in systemic hyperammonaemia after ingestion of oral probiotic EcN 1917 strain SYNB 1020 | Metabolically active cells measured in fecal arginine ↑ the clinical development of EcN 1917 strain SYNB1020 for hyperammonemia disorders, including urea cycle disorders and hepatic encephalopathy | [47] |
| Clinical trial | N = 96 Children | To evaluate the efficacy of probiotics (L. acidophilus tablets) combined with triple therapy in H.pylori infection | Triple therapy treatment and pretreated with probiotics showed better recovery of the gastric body and gastric antral mucosa | [48] |
| Randomized controlled trial | N = 88 inpatients receiving broad-spectrum antibiotics | To evaluate whether the ingestion of L. rhamnosus GG could prevent colonization or infection with AROs | L. rhamnosus GG administration neither prevented the acquisition of ARO nor accelerated the loss of ARO colonization | [49] |
| Clinical trial: randomized, double-blind | N = 329 > 18 y/o | To evaluate the effect of probiotics plus the 10-day concomitant non-bismuth quadruple H. pylori eradication regimen | LactoLevure, Uni-Pharma S.A.—Athens—Greece: ↑ the eradication rate ↓ side effects. | [50] |
| Randomized, double-blind, placebo-controlled study | N = 30 18–56 y/o | To analyze the effect of PPI-induced gastric acid suppression on the survival and colonization of a multi-strain probiotic mix (VSL Pharmaceuticals, Inc. USA, batch no: 710012) | Acid suppression enhances certain probiotic-associated bacterial colonization and probiotics in turn suppressed PPI-mediated intestinal microbial alterations. Increased microbial abundance of Streptococcaceae (p = 0.004), Leuconostacaceae (p = 0.001), and Pasteurellaceae (p = 0.020) families | [51] |
| Double-blind, randomized, placebo-controlled trial | N = 40 Adults | To evaluate the effectiveness of probiotics in reducing the bacterial load of H. pylori and modifying the gut microbiota | The use of L. acidophilus and L. rhamnosus may reduce the bacterial load of H. pylori, but no significant changes in the composition of gut microbiota | [52] |
| Randomized placebo-controlled trial | N = 120 Adults | To evaluate whether the treatment with probiotics for 10 days with amoxicillin-clavulanate antibiotics could prevent the colonization of the gut microbiota with multi-drug resistant bacteria | The probiotic mixture containing Saccharomyces boulardii, Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lpc-37, Bifidobacterium lactis Bl-04, and Bifidobacterium lactis Bi-07 led to a significant decline in colonization with Pseudomonas after antibiotic treatment | [53] |
| Randomized, double-blind, controlled trial | N = 136 Adults | To evaluate the effect of a test fermented milk containing yogurt and L. paracasei CNCM I-1518 and I-3689, L. rhamnosus CNCM I-3690 on AAD, GI symptoms, gut microbiota, and metabolites in H. pylori-infected patients | The consumption of multi-strain fermented milk can induce a modest but significantly faster recovery of the microbiota composition (beta-diversity) and SCFA production and limit the increase in potentially pathogenic bacteria. Moreover, Lacticaseibacillus strains were detected during product consumption in feces | [54] |
| Randomized controlled trial | N = 56 H. pylori-negative N = 95 H. pylori-positive subjects 19–30 y/o | To evaluate the effect of H. pylori eradication and intervention with Bifidobacterium Tetravaccine on gastric microbiota | Probiotics supplementation partially helped restore the gastric dysbiosis: Bifidobacterium was enriched in gastric mucosa, Lactobacillus was enriched in gastric juice, and Fusobacterium and Campylobacter decreased | [55] |
| Randomized controlled trial | N = 31 Adults | To determine whether the probiotic L. Rhamnosus GG prevents the colonization of the gut with multi-drug resistant bacteria in Danish travelers to India | The use of L. Rhamnosus GG did not have any effect on the risk of colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae | [56] |
| Randomized controlled trial | N = 120 < 11 y/o | To evaluate the effect on the gut microbiota of Bifidobacterium tetravaccine in children with RRTI | Oral probiotics (Bifidobacterium tetravaccine tablets) can effectively improve the RRTI intestinal micro ecological balance | [57] |
| Open-label single-center randomized parallel controlled study | N = 55 Full-term neonates | The effects of probiotics (BIFICO, Shanghai Sinepharm, China), on the gut microbiota of infectious neonates, when used concurrently with or during the recovery period following antibiotic therapy | Probiotics: did not restore the overall diversity of the gut microbiota Probiotics + antibiotics: beneficial for the gut microbiota as compared to delaying the use of probiotics to follow treatment with antibiotics | [58] |
| Clinical study | N = 60 Neonates | To characterize the probiotic potential of bacteria isolated from human neonatal feces | Selected bacteria with low pH resistance and antimicrobial activity against E. coli ATCC25922 and E. coli ATCC35218 showed probiotic potential | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda-Robles, A.; Rodríguez-Lara, A.; Meyers, M.S.; Sáez-Lara, M.J.; Álvarez-Mercado, A.I. Effect of Probiotics on Host-Microbiota in Bacterial Infections. Pathogens 2022, 11, 986. https://doi.org/10.3390/pathogens11090986
Rueda-Robles A, Rodríguez-Lara A, Meyers MS, Sáez-Lara MJ, Álvarez-Mercado AI. Effect of Probiotics on Host-Microbiota in Bacterial Infections. Pathogens. 2022; 11(9):986. https://doi.org/10.3390/pathogens11090986
Chicago/Turabian StyleRueda-Robles, Ascensión, Avilene Rodríguez-Lara, Matthew S. Meyers, María José Sáez-Lara, and Ana I. Álvarez-Mercado. 2022. "Effect of Probiotics on Host-Microbiota in Bacterial Infections" Pathogens 11, no. 9: 986. https://doi.org/10.3390/pathogens11090986