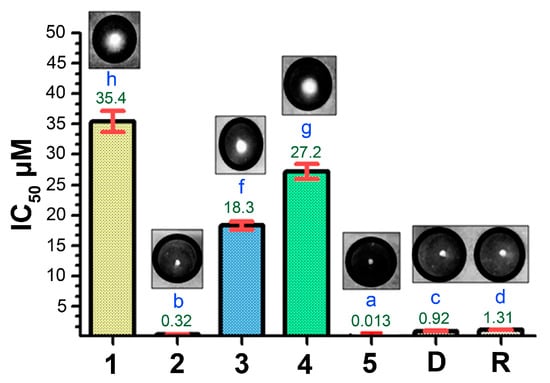
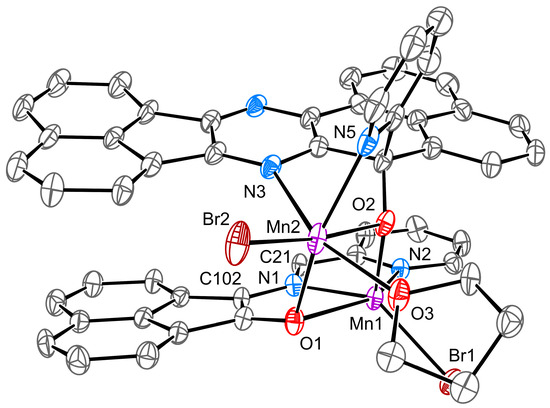
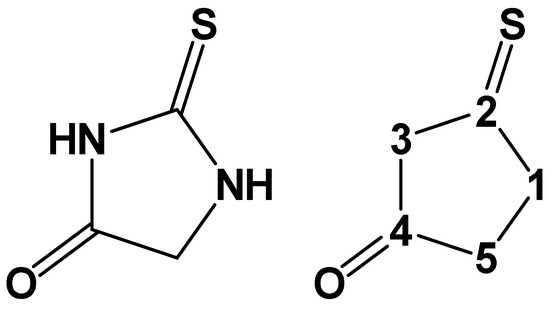
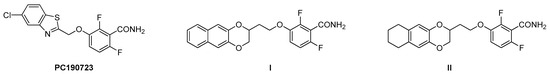
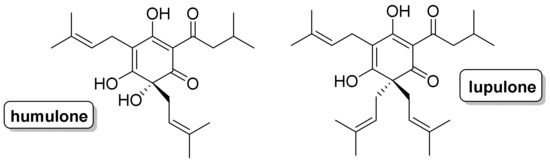
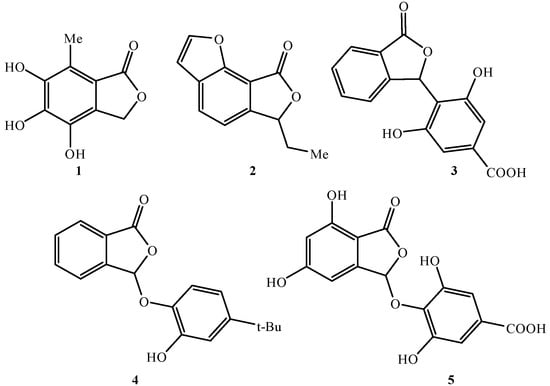
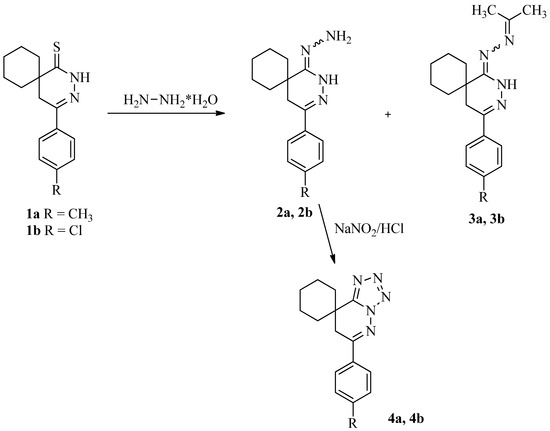
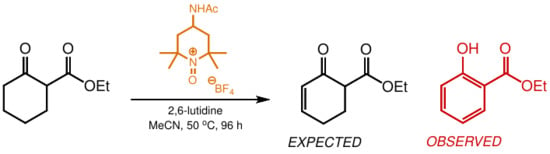
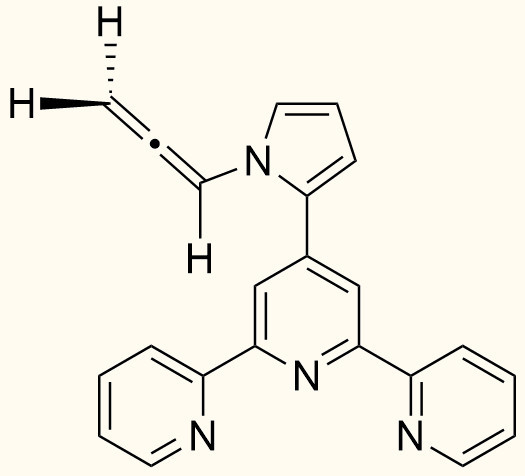
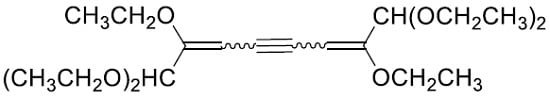
Molecules from Side Reactions
A topical collection in Molbank (ISSN 1422-8599). This collection belongs to the section "Organic Synthesis".
Viewed by 65080Editor
Interests: solid-phase synthesis; chemistry of nucleosides, nucleotides, and oligonucleotides; L-sugars; platinum complexes; heterocycles; bioorganic chemistry
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
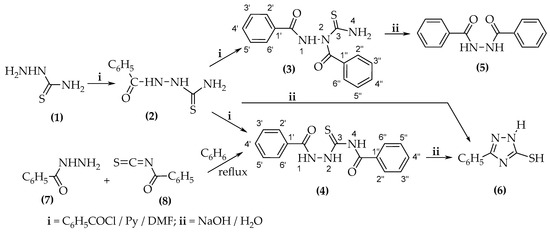
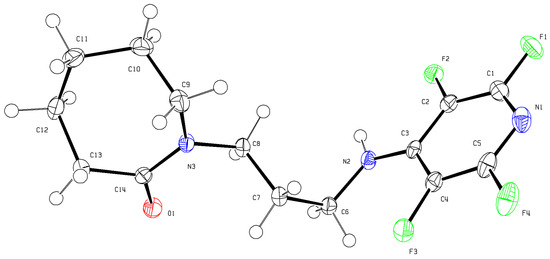
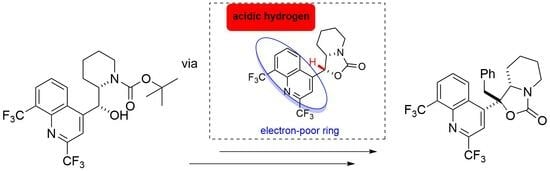
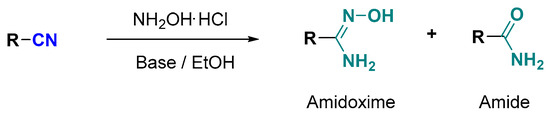
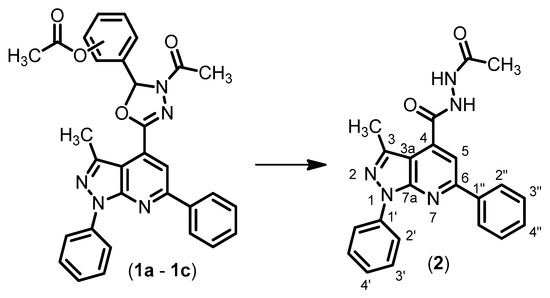
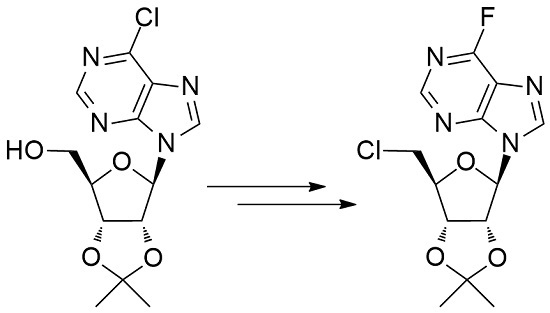
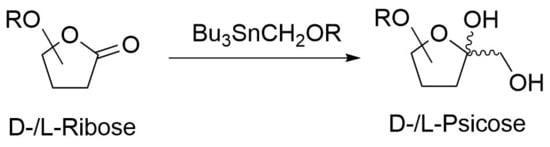
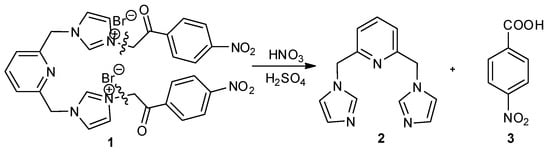
Chemical reactions are powerful tools that allow researchers to obtain novel molecular scaffolds, materials, and drugs. By fine-tuning the conditions, it is possible to drive a reaction towards the obtainment of the desired target. In addition, it is a common experience that a reaction may also follow unexpected and unpredictable routes, yielding side products. These products are usually discarded and stored in the freezers of laboratories. The aim of this Topical Collection is to share research pertaining to side products with scientists all over the world. Considering the special nature of this Topical Collection, studies addressing low yields as well as a lack of biological activities will be welcome for publication. However, side products must be fully spectroscopically characterized in accordance with the rigorous Molbank criteria.
Dr. Stefano D’Errico
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molbank is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 500 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- organic synthesis
- solid-phase synthesis
- heterocycles
- building blocks
- intermediates
- drugs
- drug design
- medicinal chemistry
- chemical diversity
- diversity-oriented synthesis
- carbon-carbon bond formation
- metal-mediated reactions
- catalyzers
Related Special Issues
- Molecules from Side Reactions II in Molecules (1 article)
- Molecules from Side Reactions II in Molbank