Retrograde Responses of Neurons and Associated Glial Cells to Axon Injury
Share This Topical Collection
Editor
Topical Collection Information
Dear Colleagues,
This Special Issue is dedicated to recent and ongoing research on the cellular and molecular framework for the retrograde neuronal reaction. Neurons and associated glial cells, particularly astrocytes and microglia, respond to axon injury (axotomy) through an integrated process, which affects the neuron’s ability to survive, regenerate and maintain its synaptic connections. Axotomized motor neurons lose a large part of their presynaptic input, “synaptic stripping”, and peripherally axotomized sensory ganglion neurons display marked alterations in their central termination territories, “transganglionic changes”. The retrograde neuronal reaction is most commonly associated with the traumatic injury of peripheral or long distance projecting central axons, but is also a frequent consequence of, for example, stroke, traumatic brain injury, invasive malignancies, and metabolic disorders or toxin exposure, which compromise axonal integrity. In all these conditions, the outcome of the retrograde neuronal reaction will have a major impact on functional recovery. This Special Issue aims to cover research on how the retrograde neuronal reaction is triggered and maintained, the mechanisms involved for neuron survival or degeneration, as well as for neuron-glial, glial-glial and neuron-neuronal interactions, and how these processes come together to disrupt the affected neural circuitry, and the possibilities for its restoration through interventions with bioactive agents and rehabilitation. We look forward to your contribution.
Dr. Hakan Aldskogius
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- neuron regeneration
- neuron degeneration
- astrocyte
- microglia
- synaptic plasticity
Published Papers (7 papers)
2022
Open AccessArticle
The Time Course of MHC-I Expression in C57BL/6J and A/J Mice Correlates with the Degree of Retrograde Gliosis in the Spinal Cord following Sciatic Nerve Crush
by
Bruno Henrique de Melo Lima, André Luis Bombeiro, Luciana Politti Cartarozzi and Alexandre Leite Rodrigues de Oliveira
Cited by 2 | Viewed by 1450
Abstract
The pleiotropic role of the major histocompatibility complex class I (MHC-I) reflects the close association between the nervous and immune systems. In turn, MHC-I upregulation postinjury is associated with a better regenerative outcome in isogenic mice following peripheral nerve damage. In the present
[...] Read more.
The pleiotropic role of the major histocompatibility complex class I (MHC-I) reflects the close association between the nervous and immune systems. In turn, MHC-I upregulation postinjury is associated with a better regenerative outcome in isogenic mice following peripheral nerve damage. In the present work, we compared the time course of neuronal, glial, and sensorimotor recovery (1, 3, 5, 7, and 28 days after lesion—dal) following unilateral sciatic nerve crush in A/J and C57BL/6J mice. The A/J strain showed higher expression of MHC-I (7 dal, **
p < 0.01), Iba-1 (microglial reaction, 7 dal, ***
p < 0.001), and GFAP (astrogliosis, 5 dal, *
p < 0.05) than the C57BL/6J counterpart. Synaptic coverage (synaptophysin) was equivalent in both strains over time. In addition, mRNA expression of microdissected spinal motoneurons revealed an increase in cytoskeleton-associated molecules (cofilin, shp2, and crmp2, *
p < 0.05), but not trkB, in C57BL/6J mice. Gait recovery, studied by the sciatic functional index, was faster in the A/J strain, despite the equivalent results of C57BL/6J at 28 days after injury. A similar recovery was also seen for the nociceptive threshold (von Frey test). Interestingly, when evaluating proprioceptive recovery, C57BL/6J animals showed an enlarged base of support, indicating abnormal ambulation postinjury. Overall, the present results reinforce the role of MHC-I expression in the plasticity of the nervous system following axotomy, which in turn correlates with the variable recovery capacity among strains of mice.
Full article
►▼
Show Figures
Open AccessArticle
Cellular Sources and Neuroprotective Roles of Interleukin-10 in the Facial Motor Nucleus after Axotomy
by
Elizabeth M. Runge, Deborah O. Setter, Abhirami K. Iyer, Eric J. Regele, Felicia M. Kennedy, Virginia M. Sanders and Kathryn J. Jones
Cited by 3 | Viewed by 2245
Abstract
Facial motoneuron (FMN) survival is mediated by CD4+ T cells in an interleukin-10 (IL-10)-dependent manner after facial nerve axotomy (FNA), but CD4+ T cells themselves are not the source of this neuroprotective IL-10. The aims of this study were to (1) identify the
[...] Read more.
Facial motoneuron (FMN) survival is mediated by CD4+ T cells in an interleukin-10 (IL-10)-dependent manner after facial nerve axotomy (FNA), but CD4+ T cells themselves are not the source of this neuroprotective IL-10. The aims of this study were to (1) identify the temporal and cell-specific induction of IL-10 expression in the facial motor nucleus and (2) elucidate the neuroprotective capacity of this expression after axotomy. Immunohistochemistry revealed that FMN constitutively produced IL-10, whereas astrocytes were induced to make IL-10 after FNA.
Il10 mRNA co-localized with microglia before and after axotomy, but microglial production of IL-10 protein was not detected. To determine whether any single source of IL-10 was critical for FMN survival, Cre/Lox mouse strains were utilized to selectively knock out IL-10 in neurons, astrocytes, and microglia. In agreement with the localization data reflecting concerted IL-10 production by multiple cell types, no single cellular source of IL-10 alone could provide neuroprotection after FNA. These findings suggest that coordinated neuronal and astrocytic IL-10 production is necessary for FMN survival and has roles in neuronal homeostasis, as well as neuroprotective trophism after axotomy.
Full article
►▼
Show Figures
Open AccessReview
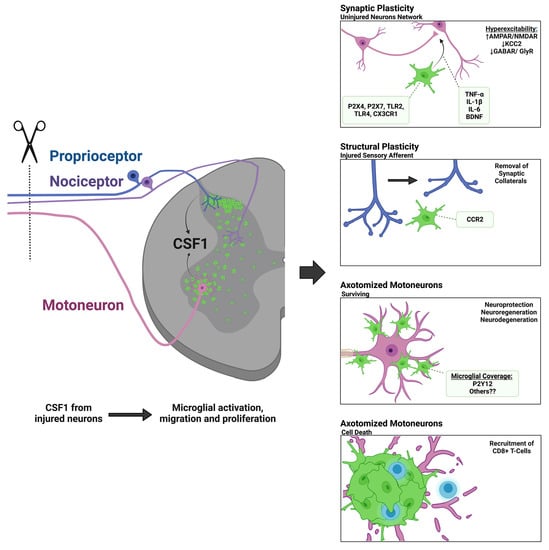
The Role of Microglia in Neuroinflammation of the Spinal Cord after Peripheral Nerve Injury
by
Tana S. Pottorf, Travis M. Rotterman, William M. McCallum, Zoë A. Haley-Johnson and Francisco J. Alvarez
Cited by 29 | Viewed by 5133
Abstract
Peripheral nerve injuries induce a pronounced immune reaction within the spinal cord, largely governed by microglia activation in both the dorsal and ventral horns. The mechanisms of activation and response of microglia are diverse depending on the location within the spinal cord, type,
[...] Read more.
Peripheral nerve injuries induce a pronounced immune reaction within the spinal cord, largely governed by microglia activation in both the dorsal and ventral horns. The mechanisms of activation and response of microglia are diverse depending on the location within the spinal cord, type, severity, and proximity of injury, as well as the age and species of the organism. Thanks to recent advancements in neuro-immune research techniques, such as single-cell transcriptomics, novel genetic mouse models, and live imaging, a vast amount of literature has come to light regarding the mechanisms of microglial activation and alluding to the function of microgliosis around injured motoneurons and sensory afferents. Herein, we provide a comparative analysis of the dorsal and ventral horns in relation to mechanisms of microglia activation (CSF1, DAP12, CCR2, Fractalkine signaling, Toll-like receptors, and purinergic signaling), and functionality in neuroprotection, degeneration, regeneration, synaptic plasticity, and spinal circuit reorganization following peripheral nerve injury. This review aims to shed new light on unsettled controversies regarding the diversity of spinal microglial-neuronal interactions following injury.
Full article
►▼
Show Figures
Open AccessReview
Events Occurring in the Axotomized Facial Nucleus
by
Kazuyuki Nakajima and Takashi Ishijima
Cited by 4 | Viewed by 1909
Abstract
Transection of the rat facial nerve leads to a variety of alterations not only in motoneurons, but also in glial cells and inhibitory neurons in the ipsilateral facial nucleus. In injured motoneurons, the levels of energy metabolism-related molecules are elevated, while those of
[...] Read more.
Transection of the rat facial nerve leads to a variety of alterations not only in motoneurons, but also in glial cells and inhibitory neurons in the ipsilateral facial nucleus. In injured motoneurons, the levels of energy metabolism-related molecules are elevated, while those of neurofunction-related molecules are decreased. In tandem with these motoneuron changes, microglia are activated and start to proliferate around injured motoneurons, and astrocytes become activated for a long period without mitosis. Inhibitory GABAergic neurons reduce the levels of neurofunction-related molecules. These facts indicate that injured motoneurons somehow closely interact with glial cells and inhibitory neurons. At the same time, these events allow us to predict the occurrence of tissue remodeling in the axotomized facial nucleus. This review summarizes the events occurring in the axotomized facial nucleus and the cellular and molecular mechanisms associated with each event.
Full article
►▼
Show Figures
Open AccessArticle
Chronic Chemogenetic Activation of the Superior Colliculus in Glaucomatous Mice: Local and Retrograde Molecular Signature
by
Marie Claes, Emiel Geeraerts, Stéphane Plaisance, Stephanie Mentens, Chris Van den Haute, Lies De Groef, Lut Arckens and Lieve Moons
Cited by 5 | Viewed by 2861
Abstract
One important facet of glaucoma pathophysiology is axonal damage, which ultimately disrupts the connection between the retina and its postsynaptic brain targets. The concurrent loss of retrograde support interferes with the functionality and survival of the retinal ganglion cells (RGCs). Previous research has
[...] Read more.
One important facet of glaucoma pathophysiology is axonal damage, which ultimately disrupts the connection between the retina and its postsynaptic brain targets. The concurrent loss of retrograde support interferes with the functionality and survival of the retinal ganglion cells (RGCs). Previous research has shown that stimulation of neuronal activity in a primary retinal target area—i.e., the superior colliculus—promotes RGC survival in an acute mouse model of glaucoma. To build further on this observation, we applied repeated chemogenetics in the superior colliculus of a more chronic murine glaucoma model—i.e., the microbead occlusion model—and performed bulk RNA sequencing on collicular lysates and isolated RGCs. Our study revealed that chronic target stimulation upon glaucomatous injury phenocopies the a priori expected molecular response: growth factors were pinpointed as essential transcriptional regulators both in the locally stimulated tissue and in distant, unstimulated RGCs. Strikingly, and although the RGC transcriptome revealed a partial reversal of the glaucomatous signature and an enrichment of pro-survival signaling pathways, functional rescue of injured RGCs was not achieved. By postulating various explanations for the lack of RGC neuroprotection, we aim to warrant researchers and drug developers for the complexity of chronic neuromodulation and growth factor signaling.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
Satellite Glial Cells and Neurons in Trigeminal Ganglia Are Altered in an Itch Model in Mice
by
Meytal Cohen, Rachel Feldman-Goriachnik and Menachem Hanani
Cited by 3 | Viewed by 2512
Abstract
Itch (pruritus) is a common chronic condition with a lifetime prevalence of over 20%. The mechanisms underlying itch are poorly understood, and its therapy is difficult. There is recent evidence that following nerve injury or inflammation, intercellular communications in sensory ganglia are augmented,
[...] Read more.
Itch (pruritus) is a common chronic condition with a lifetime prevalence of over 20%. The mechanisms underlying itch are poorly understood, and its therapy is difficult. There is recent evidence that following nerve injury or inflammation, intercellular communications in sensory ganglia are augmented, which may lead to abnormal neuronal activity, and hence to pain, but there is no information whether such changes take place in an itch model. We studied changes in neurons and satellite glial cells (SGCs) in trigeminal ganglia in an itch model in mice using repeated applications of 2,4,6-trinitro-1-chlorobenzene (TNCB) to the external ear over a period of 11 days. Treated mice showed augmented scratching behavior as compared with controls during the application period and for several days afterwards. Immunostaining for the activation marker glial fibrillary acidic protein in SGCs was greater by about 35% after TNCB application, and gap junction-mediated coupling between neurons increased from about 2% to 13%. The injection of gap junction blockers reduced scratching behavior, suggesting that gap junctions contribute to itch. Calcium imaging studies showed increased responses of SGCs to the pain (and presumed itch) mediator ATP. We conclude that changes in both neurons and SGCs in sensory ganglia may play a role in itch.
Full article
►▼
Show Figures
Open AccessReview
How Is Peripheral Injury Signaled to Satellite Glial Cells in Sensory Ganglia?
by
Menachem Hanani
Cited by 10 | Viewed by 3965
Abstract
Injury or inflammation in the peripheral branches of neurons of sensory ganglia causes changes in neuronal properties, including excessive firing, which may underlie chronic pain. The main types of glial cell in these ganglia are satellite glial cells (SGCs), which completely surround neuronal
[...] Read more.
Injury or inflammation in the peripheral branches of neurons of sensory ganglia causes changes in neuronal properties, including excessive firing, which may underlie chronic pain. The main types of glial cell in these ganglia are satellite glial cells (SGCs), which completely surround neuronal somata. SGCs undergo activation following peripheral lesions, which can enhance neuronal firing. How neuronal injury induces SGC activation has been an open question. Moreover, the mechanisms by which the injury is signaled from the periphery to the ganglia are obscure and may include electrical conduction, axonal and humoral transport, and transmission at the spinal level. We found that peripheral inflammation induced SGC activation and that the messenger between injured neurons and SGCs was nitric oxide (NO), acting by elevating cyclic guanosine monophosphate (cGMP) in SGCs. These results, together with work from other laboratories, indicate that a plausible (but not exclusive) mechanism for neuron-SGCs interactions can be formulated as follows: Firing due to peripheral injury induces NO formation in neuronal somata, which diffuses to SGCs. This stimulates cGMP synthesis in SGCs, leading to their activation and to other changes, which contribute to neuronal hyperexcitability and pain. Other mediators such as proinflammatory cytokines probably also contribute to neuron-SGC communications.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: spinal cord injury and multiple sclerosis
Authors: Stella Elkabes
Affiliation: Cancer Research Center (CANCT) 195 South Orange Avenue Room F 1204 Rutgers New Jersey Medical School , Newark, United States
Title: Bilateral Phasic Alterations in Luman/CREB3 and Downstream UPR Target Expression in Unilaterally Injured Rat Motor Neurons: Acceleration and Alteration of Response by Electrical Nerve Stimulation
Authors: Olivia F. Woo1,2, Emma Russell1,2, Jayne M. Johnston1,2, Tessa Gordon3 and Valerie M. K. Verge1,2
Affiliation: 1Department of Anatomy, Physiology and Pharmacology, University of Saskatchewan, Saskatoon, SK, Canada S7N 5E5.
2Cameco MS Neuroscience Research Centre, University of Saskatchewan, Saskatoon, Saskatchewan Canada S7K 0M7.
3SickKids Research Institute, Neuroscience and Mental Health Program; Division of Plastic and Reconstructive Surgery, the Hospital for Sick Children, Toronto, ON, Canada
Abstract: Luman./CREB3 is an important retrograde signal linked to sensory axon regeneration through its regulation of the unfolded protein response (UPR). Temporal Luman/CREB3 expression in injured sensory neurons is biphasic, with peaks correlating with heightened regenerative states in the first week of injury with parallel responses in neurons remote and contralateral to injury. Whether motor neurons express Luman/CREB3 and undergo similar phasic alterations in Luman/CREB3 and UPR-associated downstream target expression following injury and whether this is altered by brief electrical nerve stimulation (ES) which greatly improves motor axon regeneration are unknown. Thus, temporal analysis of Luman/CREB3 and downstream target expression at 0, 1,2, 4, 7 and 10 days post unilateral sciatic nerve crush in male rats+/- 1 hour ES at time of injury was assessed in motor neurons using immunofluorescence analysis. Results reveal monophasic cytoplasmic and nuclear Luman/CREB3 and cytoplasmic Bip/Grp78 expression peaking at 2 days in injured and uninjured motor neurons. ES resulted in an accelerated biphasic response, peaking at 1 day post injury with a subsequent peak at 10 days in injured and uninjured neurons. A similar pattern of response was observed for the UPR marker CHOP not in the motor neurons but in the surrounding neuropil. Collectively, this supports that ES-induced accelerated and enhanced biphasic expression of Luman/CREB3 and downstream UPR markers may factor into the accelerated and enhanced regeneration observed in motor neurons in response to ES.
Title: The many faces of spinal microglia and their functional diversity after nerve injury
Authors: Alvarez, Francisco
Affiliation: Department of Cell Biology
Emory University School of Medicine
Whitehead Research Building, Room 642
615 Michael Street
Atlanta, GA, 30322-3110
Office phone: 404-727-5139
Abstract: a review for Cells on the microgliosis that occurs in the spinal cord after peripheral nerve injury. We will emphasize the variety of microglia activation states and functions that can be found in the spinal cord after a peripheral nerve injury and the variety effector mechanisms. For that reason we will like to entitle this review as follows.
Title: Chronic chemogenetic activation of the superior colliculus in glaucomatous mice: local and retrograde effects.
Authors: Marie Claes, Stéphane Plaisance, Chris Van den Haute, Lies De Groef, Lut Arckens and Lieve Moons
Affiliation: Neural Circuit Development and Regeneration Research Group, Department of Biology, University of Leuven
Abstract: An important facet of glaucoma pathology is axonal injury, ultimately disrupting the tie between the retina and its postsynaptic visual nuclei. Concurrent loss of target-derived support possibly interferes with the functionality and survival of the retinal ganglion cells (RGCs). By optogenetically stimulating neuronal activity in a postsynaptic target, the superior colliculus, we previously promoted RGC survival in an experimental mouse model of glaucoma. Strikingly, using chemogenetics (vs. optogenetics) and a chronic (vs. acute) glaucoma model, functional rescue of injured RGCs could not be achieved. Bulk RNA sequencing of isolated RGCs and collicular lysates pinpointed growth factors as essential upstream regulators upon chronic target stimulation in glaucomatous mice. However, and although exerting well-known neuroprotective functions, these factors seem to lose the battle against the glaucomatous insults. Various explanations for the lack of RGC rescue are postulated by critically discussing our experimental set-up and bidirectional biological actions of the predicted upstream regulators. Even though RGC rescue from glaucomatous injury was not achieved, the transcriptomic profile changes upon chronic chemogenetic stimulation in both locally stimulated and distant unstimulated neurons significantly contribute to the chemogenetic research field, in which the molecular underpinning of neuromodulation is lacking.