Role of Stromal Cells in Determining Tumor and Cancer Stem Cell Behaviors and Therapeutic Response
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Molecular Cancer Biology".
Viewed by 46213Editors
Interests: cancer pathophysiology; tumor microenvironment; pH regulation; invasion; vasculogenesis; hypoxia; breast; pancreatic; prostate
Interests: role of tumor microenvironment (TME) in driving PDAC; mechanisms of PDAC metastasis; mechanisms of PDAC therapy resistance; novel chemo- and immunotherapies for PDAC
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
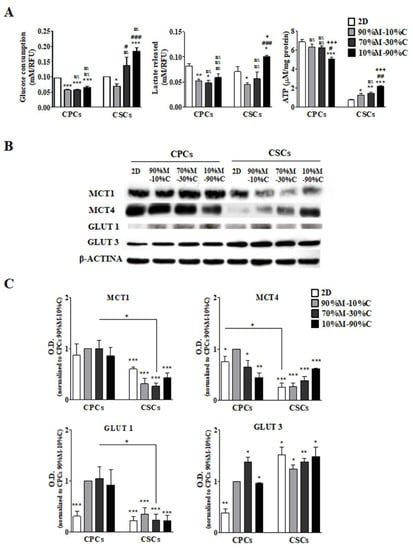
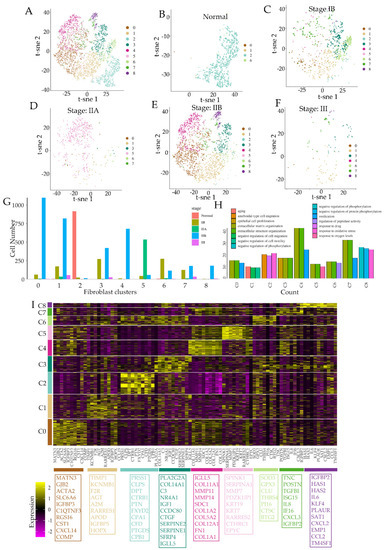
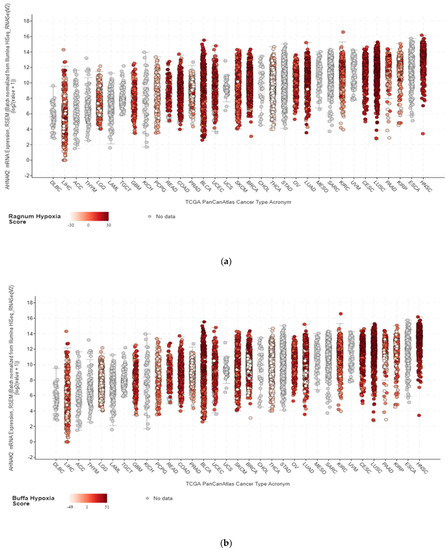
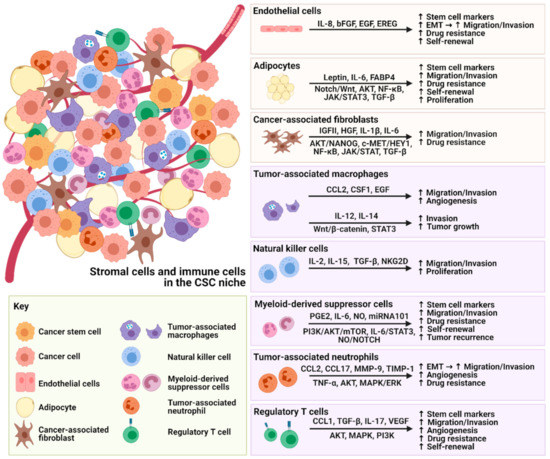
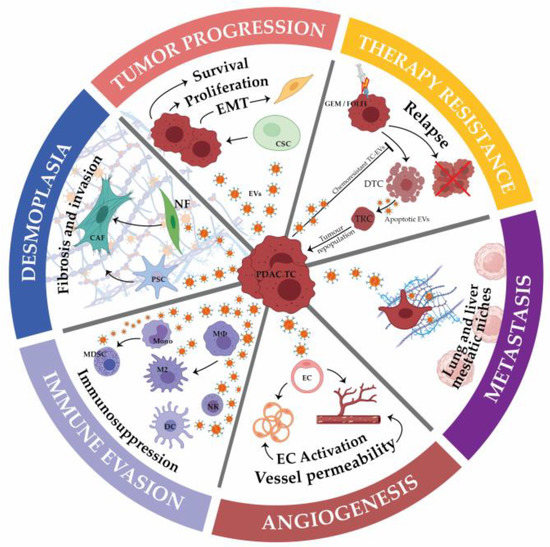
The tumor microenvironment (TME) is composed of stromal extracellular matrix (ECM) components (i.e. laminins, fibronectin, collagens, proteoglycans, elastin) and stromal cells (fibroblasts, endothelial cells, macrophages and lymphocytes). Most of these cellular components produce tumor-supportive ECM and secrete growth factors and chemokines that further alter theECM and generate oncogenic signals, thus playing key roles in tumor transformation, cell proliferation and tissue invasion. Tumor ECM is altered during malignant progression, and its interaction with cancer cells plays an essential role in tumor metabolism, development, progression, recruitment and metabolic reprograming of tumor and stromal cells and treatment response. The TME also includes the tumor metabolic microenvironment (TMM), which is characterized by dynamic, interacting areas of hypoxia, low extracellular pH (pHe) and low nutrients. This adverse pathophysiological TMM is formed by vascular abnormalities, inadequate microcirculation, high vascular permeability and increased interstitial fluid pressure. Beyond hypoxia and acidic pHe, this TMM leads to the upregulation of glycolytic capacity (Warburg effect), lactate accumulation and energy depletion. Of these conditions, the best characterized is hypoxia, which contributes, among other effects, to mutagenesis, suppression of apoptosis, epithelial-to-mesenchymal transition and cancer stem cell (CSC) selection. Highly acidic TME (pHe 6.4-6.8) is the result of elevated metabolic rates in highly proliferative cancer cells, in conjunction with often greatly increased rates of net cellular acid extrusion.
Indeed, the acidic tumor microenvironment is an important contributing factor to the metastasis of cancer cells, via a combination of toxicity to adjacent normal cells, degradation of the ECM through the induced secretion and activation of proteases, increased cancer cell motility and invasion, reduced immunological defenses and stromal cells activation, and/or recruitment to drive malignant progression.
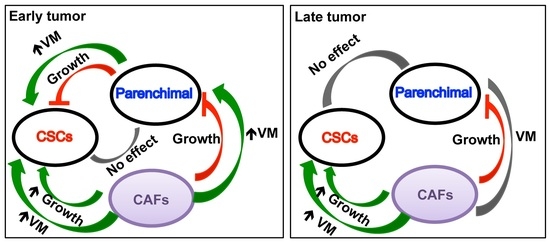
The reciprocal interactions of stromal cell types with neoplastic cells (the bulk parenchymal tumor cells and CSCs), the ECM and the metabolic microenvironment are highly complex and most likely vary between tumor types and stages. Given the highly complex nature of the tumor microenvironment and its components, it is likely that many microenvironmental factors contribute to cancer growth and progression, and that they interact and co-evolve in very complex ways.
The challenge, therefore, is to identify the important aspects of these interactions in the context of metastatic progression. This Topical Collection will present research papers and reviews, exploring the complexities and roles of stromal cells in determining the heterogeneity of tumor properties and behaviors, to improve our understanding of metastatic progression and therapeutic resistance.
Dr. Stephan Joel Reshkin
Dr. Rosa Angela Cardone
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- stromal cells
- cancer stem cells
- tumor stromal and biological microenvironments
- spatial intratumoral pH
- pO2
- and nutrient concentrations