Wound Fluid from Breast Cancer Patients Undergoing Intraoperative Radiotherapy Exhibits an Altered Cytokine Profile and Impairs Mesenchymal Stromal Cell Function
Abstract
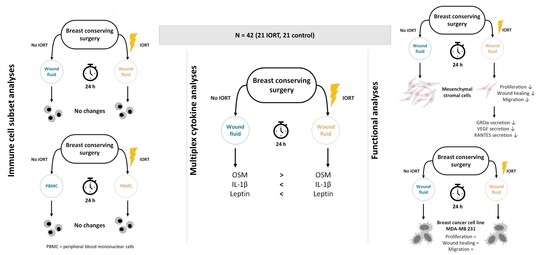
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection
2.3. Flow Cytometric Analysis
2.4. Multiplex Cytokine Analysis
2.5. Cell Culture
2.6. Effects of Wound Fluid on Cell Function
- Proliferation assay. MDA-MB 231 and MSC were seeded at a density of 2 × 103 cells per well and cultivated at 37 °C, 5% CO2 for 24 h. MDA-MB 231 cells were cultured for additional 24 h in serum-free medium. Subsequently, the culture medium was replaced by WF-containing media. Cell proliferation was monitored using the IncuCyte ZOOM® system and quantified as percent confluence using either phase contrast (MSC) or nuclear GFP (MDA-MB 231) values.
- Wound healing/scratch assay. MDA-MB 231 and MSC were seeded at a density of 2 × 104 cells per well and incubated at 37 °C, 5% CO2 for 18 h. MDA-MB 231 cells were starved then for 24 h in serum-free medium. Afterwards, a scratch wound was set to the confluent monolayer (IncuCyte WoundMakerTM, Sartorius, Hertfordshire, United Kingdom). The cells were washed twice and WF containing media was added. Wound healing was monitored for 48 h. For both cell types, wound healing related migration was determined using phase contrast.
- Migration/chemotaxis assay. The insert plate of a IncuCyte ClearView 96 well plate (Sartorius, Hertfordshire, United Kingdom) was coated with fibronectin. Subsequently, MDA-MB 231 and MSC were seeded at a density of 1 × 103 cells per well in DMEM + 1% HS or FBS; respectively. After settling for 1 h at ambient temperature, the medium was replaced with serum-free DMEM. The insert plate was mated with the reservoir plate loaded with WF-containing media. Cell migration to the reservoir plate was monitored for 48 h). For both cell types, chemotactic migration was determined using phase contrast.
2.7. Collection of Conditioned Media
2.8. Statistical Methods
3. Results
3.1. Immune Cell Subpopulations, Their Activation and Apoptosis State Are Not Changed in Wound Fluid from IORT-Treated Patients
3.2. Wound Fluid from IORT-Treated Patients Exhibits an Altered Cytokine Profile
3.3. Wound Fluid from IORT-Treated Patients Affects MSC Behavior
3.4. Wound Fluid from IORT-Treated Patients Modifies the Secretome of MSC
4. Discussion
4.1. Immune Cell Subpopulations, Their Activation and Apoptosis State Are Not Changed in Wound Fluid from IORT-Treated Patients
4.2. Wound Fluid from IORT-Treated Patients Exhibits an Altered Cytokine Profile
4.3. Wound Fluid from IORT-Treated Patients Affects MSC, but not MDA-MB 231, Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| °C | degree Celsius |
| BCS | breast conserving surgery |
| CD | cluster of differentiation |
| cm2 | square centimetres |
| CO2 | carbon dioxide |
| CSC | cancer stem cells |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| EBRT | external beam radiotherapy |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| EMT | epithelial-mesenchymal transition |
| et al. | et alia |
| FBS | foetal bovine serum |
| FGF | fibroblast growth factor |
| GFP | green fluorescent protein |
| GROα | growth-regulated oncogene alpha |
| Gy | Gray |
| h | hours |
| HGF | hepatocyte growth factor |
| HS | human serum |
| IL-1β | interleukin 1 beta |
| IORT | intraoperative radiotherapy |
| MANOVA | multivariate variance analysis |
| MSC | mesenchymal stromal cells |
| N | number of biological replicates |
| n | number of technical replicates |
| NST | no special type |
| OSM | oncostatin-M |
| p | p value |
| P | cell passage |
| PBMC | peripheral blood mononuclear cells |
| PDGF | platelet derived growth factor |
| RANTES | regulated and normal T cell expressed and secreted, also: CCL5 |
| SD | standard deviation |
| STAT3 | signal transducer and activator of transcription 3 |
| TNBC | triple negative breast cancer |
| Treg | regulatory T cells |
| uPA | urokinase-type plasminogen activator |
| VEGF | vascular endothelial growth factor |
| WF | wound fluid |
References
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef]
- Darby, S.C.; McGale, P.; Correa, C.R.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, J.M.; Amalric, R.; Brandone, H.; Ayme, Y.; Jacquemier, J.; Pietra, J.-C.; Hans, D.; Pollet, J.-F.; Bressac, C.; Spitalier, J.-M. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer 1989, 63, 1912–1917. [Google Scholar] [CrossRef]
- Veronesi, U.; Luini, A.; Del Vecchio, M.; Greco, M.; Galimberti, V.; Merson, M.; Rilke, F.; Sacchini, V.; Saccozzi, R.; Savio, T.; et al. Radiotherapy after Breast-Preserving Surgery in Women with Localized Cancer of the Breast. N. Engl. J. Med. 1993, 328, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol. 2014, 89, 16–26. [Google Scholar] [CrossRef]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3464. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.; Schwertschkow, A.H.; Nolta, J.; Wu, J. Involvement of Mesenchymal Stem Cells in Cancer Progression and Metastases. Curr. Cancer Drug Targets 2015, 15, 88–98. [Google Scholar] [CrossRef]
- Formenti, S.C.; DeMaria, S. Local control by radiotherapy: Is that all there is? Breast Cancer Res. 2008, 10, 215–217. [Google Scholar] [CrossRef]
- Blank, E.; Kraus-Tiefenbacher, U.; Welzel, G.; Keller, A.; Bohrer, M.; Sütterlin, M.; Wenz, F. Single-Center Long-Term Follow-Up After Intraoperative Radiotherapy as a Boost During Breast-Conserving Surgery Using Low-Kilovoltage X-Rays. Ann. Surg. Oncol. 2010, 17, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.S.; Baum, M.; Tobias, J.S.; Wenz, F.; Massarut, S.; Keshtgar, M.; Hilaris, B.; Saunders, C.; Williams, N.R.; Brew-Graves, C.; et al. Long-Term Results of Targeted Intraoperative Radiotherapy (Targit) Boost During Breast-Conserving Surgery. Int. J. Radiat. Oncol. 2011, 81, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Wenz, F.; Welzel, G.; Blank, E.; Hermann, B.; Steil, V.; Sütterlin, M.; Kraus-Tiefenbacher, U. Intraoperative Radiotherapy as a Boost During Breast-Conserving Surgery Using Low-Kilovoltage X-Rays: The First 5 Years of Experience With a Novel Approach. Int. J. Radiat. Oncol. 2010, 77, 1309–1314. [Google Scholar] [CrossRef]
- Kraus-Tiefenbacher, U.; Bauer, L.; Scheda, A.; Fleckenstein, K.; Keller, A.; Herskind, C.; Steil, V.; Melchert, F.; Wenz, F. Long-term toxicity of an intraoperative radiotherapy boost using low energy X-rays during breast-conserving surgery. Int. J. Radiat. Oncol. 2006, 66, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Pez, M.; Keller, A.; Welzel, G.; Abo-Madyan, Y.; Ehmann, M.; Tuschy, B.; Berlit, S.; Sütterlin, M.; Wenz, F.; Giordano, F.A.; et al. Long-term outcome after intraoperative radiotherapy as a boost in breast cancer. Strahlenther Onkol. 2019, 196, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.H.M.; Speijer, G.; Petoukhova, A.L.; Roeloffzen, E.M.A.; Straver, M.; Marinelli, A.; Fisscher, U.; Zwanenburg, A.G.; Merkus, J.; Marijnen, C.A.M.; et al. Acute toxicity of intraoperative radiotherapy and external beam-accelerated partial breast irradiation in elderly breast cancer patients. Breast Cancer Res. Treat. 2018, 169, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus-Tiefenbacher, U.; Bauer, L.; Kehrer, T.; Hermann, B.; Melchert, F.; Wenz, F. Intraoperative Radiotherapy (IORT) as a Boost in Patients with Early-Stage Breast Cancer—Acute Toxicity. Oncol. Res. Treat. 2006, 29, 77–82. [Google Scholar] [CrossRef]
- Son, B.; Lee, S.; Youn, H.; Kim, E.; Kim, W.; Youn, B. The role of tumor microenvironment in therapeutic resistance. Oncotarget 2016, 8, 3933–3945. [Google Scholar] [CrossRef] [Green Version]
- Wersal, C.; Keller, A.; Weiss, C.; Giordano, F.A.; Abo-Madyan, Y.; Tuschy, B.; Sütterlin, M.; Wenz, F.; Sperk, E. Long-term changes in blood counts after intraoperative radiotherapy for breast cancer—single center experience and review of the literature. Transl. Cancer Res. 2019, 8, 1882–1903. [Google Scholar] [CrossRef]
- Uhlig, S.; Wuhrer, A.; Berlit, S.; Tuschy, B.; Sütterlin, M.; Bieback, K. Intraoperative radiotherapy for breast cancer treatment effi-ciently targets the tumor bed preventing breast adipose stromal cell outgrowth. Strahlenther Onkol. 2020, 196, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Fabris, L.; Berton, S.; Citron, F.; D’Andrea, S.; Segatto, I.; Nicoloso, M.S.; Massarut, S.; Armenia, J.; Zafarana, G.; Rossi, S.; et al. Radiotherapy-induced miR-223 prevents relapse of breast cancer by targeting the EGF pathway. Oncogene 2016, 35, 4914–4926. [Google Scholar] [CrossRef] [PubMed]
- Belletti, B.; Vaidya, J.S.; D’Andrea, S.; Entschladen, F.; Roncadin, M.; Lovat, F.; Berton, S.; Perin, T.; Candiani, E.; Reccanello, S.; et al. Targeted Intraoperative Radiotherapy Impairs the Stimulation of Breast Cancer Cell Proliferation and Invasion Caused by Surgical Wounding. Clin. Cancer Res. 2008, 14, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, I.; Kulcenty, K.; Murawa, D.; Suchorska, W. Surgical wound fluids from patients treated with intraoperative radio-therapy induce radiobiological response in breast cancer cells. Med. Oncol. 2018, 36, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulcenty, K.; Piotrowski, I.; Wróblewska, J.P.; Wasiewicz, J.; Suchorska, A.W.M. The Composition of Surgical Wound Fluids from Breast Cancer Patients is Affected by Intraoperative Radiotherapy Treatment and Depends on the Molecular Subtype of Breast Cancer. Cancers 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulcenty, K.; Piotrowski, I.; Zaleska, K.; Wichtowski, M.; Wróblewska, J.; Murawa, D.; Suchorska, W.M. Wound fluids collected postoperatively from patients with breast cancer induce epithelial to mesenchymal transition but intraoperative radiotherapy impairs this effect by activating the radiation-induced bystander effect. Sci. Rep. 2019, 9, 7891. [Google Scholar] [CrossRef]
- Kulcenty, K.; Piotrowski, I.; Rucinski, M.; Wroblewska, J.P.; Jopek, K.; Murawa, D.; Suchorska, W.M. Surgical Wound Fluids from Patients with Breast Cancer Reveal Similarities in the Biological Response Induced by Intraoperative Radiation Therapy and the Radiation-Induced Bystander Effect—Transcriptomic Approach. Int. J. Mol. Sci. 2020, 21, 1159. [Google Scholar] [CrossRef] [Green Version]
- Sütterlin, M.; Sperk, E.; Berlit, S.; Wenz, F.; Tuschy, B. Intraoperative Strahlentherapie beim Mammakarzinom mit niederenergetischen Röntgenstrahlen. Senologie-Zeitschrift für Mammadiagnostik und-therapie 2015, 12, 150–154. [Google Scholar] [CrossRef]
- van Engeland, M.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Carpentier, G.; Henault, E. Protein array analyzer for ImageJ. In Proceedings of the ImageJ User and Developer Conference, Mondorf-les-Bains, Luxembourg, 27–29 October 2010; pp. 238–240. [Google Scholar]
- Baldassarre, G.; Belletti, B.; Vaidya, J.S.; D’Andrea, S.; Roncadin, M.; Perin, T.; Trova, M.G.; Candiani, E.; Veronesi, A.; Colombatti, A.; et al. Intraoperative radiotherapy (IORT) impairs surgical wound-stimulated breast cancer cell invasion. J. Clin. Oncol. 2007, 25, 21139. [Google Scholar] [CrossRef]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]
- Mandel, K.; Yang, Y.; Schambach, A.; Glage, S.; Otte, A.; Hass, R. Mesenchymal Stem Cells Directly Interact with Breast Cancer Cells and Promote Tumor Cell Growth In Vitro and In Vivo. Stem Cells Dev. 2013, 22, 3114–3127. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Watkins, G.; Mansel, R.E.; Jiang, W.G. The Hepatocyte Growth Factor Regulatory Factors in Human Breast Cancer. Clin. Cancer Res. 2004, 10, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nat. Cell Biol. 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Park, T.S.; Zambidis, E.T.; Donnenberg, V.S.; Donnenberg, A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013, 95, 2235–2245. [Google Scholar] [CrossRef]
- Linares-Galiana, I.; Berenguer-Frances, M.A.; Cañas-Cortés, R.; Pujol-Canadell, M.; Comas-Antón, S.; Martínez, E.; Laplana, M.; Pérez-Montero, H.; Pla-Farnós, M.J.; Navarro-Martin, A.; et al. Changes in peripheral immune cells after intraoperative radiation therapy in low-risk breast cancer. J. Radiat. Res. 2021, 62, 110–118. [Google Scholar] [CrossRef]
- Schauer, D.; Starlinger, P.; Zajc, P.; Alidzanovic, L.; Maier, T.; Buchberger, E.; Pop, L.; Gruenberger, B.; Gruenberger, T.; Brostjan, C. Monocytes with angiogenic potential are selectively induced by liver resection and accumulate near the site of liver regeneration. BMC Immunol. 2014, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- de la Cruz-Merino, L.; Illescas-Vacas, A.; Grueso-López, A.; Barco-Sánchez, A.; Míguez-Sánchez, C.; Group, C.I. Radiation for awakening the dormant immune system, a promising challenge to be explored. Front. Immunol. 2014, 5, 102. [Google Scholar] [CrossRef] [Green Version]
- DeMaria, S.; Formenti, S.C. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int. J. Radiat. Biol. 2007, 83, 819–825. [Google Scholar] [CrossRef]
- Shiao, S.L.; Coussens, L.M. The Tumor-Immune Microenvironment and Response to Radiation Therapy. J. Mammary Gland. Biol. Neoplasia 2010, 15, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapeire, L.; Hendrix, A.; Lambein, K.; Van Bockstal, M.; Braems, G.; Broecke, R.V.D.; Limame, R.; Mestdagh, P.; Vandesompele, J.; Vanhove, C.; et al. Cancer-Associated Adipose Tissue Promotes Breast Cancer Progression by Paracrine Oncostatin M and Jak/STAT3 Signaling. Cancer Res. 2014, 74, 6806–6819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, N.; Murphy, L.C.; Watson, P.H. Oncostatin M suppresses oestrogen receptor-α expression and is associated with poor outcome in human breast cancer. Endocr. Relat. Cancer 2012, 19, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Holzer, R.G.; Ryan, R.E.; Tommack, M.; Schlekeway, E.; Jorcyk, C.L. Oncostatin M stimulates the detachment of a reservoir of invasive mammary carcinoma cells: Role of cyclooxygenase-2. Clin. Exp. Metastasis 2004, 21, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Junk, D.J.; Bryson, B.L.; Smigiel, J.M.; Parameswaran, N.; Bartel, C.; Jackson, M.W. Oncostatin M promotes cancer cell plasticity through cooperative STAT3-SMAD3 signaling. Oncogene 2017, 36, 4001–4013. [Google Scholar] [CrossRef] [Green Version]
- West, N.R.; Murray, J.; Watson, P.H. Oncostatin-M promotes phenotypic changes associated with mesenchymal and stem cell-like differentiation in breast cancer. Oncogene 2014, 33, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Jirillo, E.; Matarese, G. Leptin as an immunomodulator. Mol. Asp. Med. 2012, 33, 35–45. [Google Scholar] [CrossRef]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
- Grossmann, M.E.; Ray, A.; Nkhata, K.J.; Malakhov, D.A.; Rogozina, O.P.; Dogan, S.; Cleary, M.P. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010, 29, 641–653. [Google Scholar] [CrossRef]
- Tulotta, C.; Ottewell, P.D. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer 2018, 25, R421–R434. [Google Scholar] [CrossRef] [Green Version]
- Castaño, Z.; San Juan, B.P.; Spiegel, A.; Pant, A.; DeCristo, M.J.; Laszewski, T.; Ubellacker, J.M.; Janssen, S.R.; Dongre, A.; Reinhardt, F.; et al. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat. Cell Biol. 2018, 20, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Škoberne, M.; Beignon, A.-S.; Bhardwaj, N. Danger signals: A time and space continuum. Trends Mol. Med. 2004, 10, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.R.; Weaver, A.M.; Cummings, P.T.; Quaranta, V. Tumor Morphology and Phenotypic Evolution Driven by Selective Pressure from the Microenvironment. Cell 2006, 127, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, J.; Brugge, J.S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer 2005, 5, 675–688. [Google Scholar] [CrossRef]
- Hung, S.-C.; Pochampally, R.R.; Chen, S.-C.; Hsu, S.-C.; Prockop, D.J. Angiogenic Effects of Human Multipotent Stromal Cell Conditioned Medium Activate the PI3K-Akt Pathway in Hypoxic Endothelial Cells to Inhibit Apoptosis, Increase Survival, and Stimulate Angiogenesis. STEM CELLS 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- Beegle, J.R.; Magner, N.L.; Kalomoiris, S.; Harding, A.; Zhou, P.; Nacey, C.; White, J.L.; Pepper, K.; Gruenloh, W.; Annett, G.; et al. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol. Ther. Methods Clin. Dev. 2016, 3, 16053. [Google Scholar] [CrossRef]
- Devalaraja, R.M.; Nanney, L.B.; Qian, Q.; Du, J.; Yu, Y.; Devalaraja, M.N.; Richmond, A. Delayed Wound Healing in CXCR2 Knockout Mice. J. Investig. Dermatol. 2000, 115, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Haghnegahdar, H.; Du, J.; Wang, D.; Strieter, R.M.; Burdick, M.D.; Nanney, L.B.; Cardwell, N.; Luan, J.; Shattuck-Brandt, R.; Richmond, A. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J. Leukoc. Biol. 2000, 67, 53–62. [Google Scholar] [CrossRef]
- Gallo, M.; Frezzetti, D.; Roma, C.; Chicchinelli, N.; Barbieri, A.; Arra, C.; Scognamiglio, G.; Botti, G.; De Luca, A.; Normanno, N. RANTES and IL-6 cooperate in inducing a more aggressive phenotype in breast cancer cells. Oncotarget 2018, 9, 17543–17553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, K.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. GROalpha overexpression drives cell migration and invasion in triple negative breast cancer cells. Oncol. Rep. 2017, 38, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaleska, K.; Przybyła, A.; Kulcenty, K.; Wichtowski, M.; Mackiewicz, A.; Suchorska, W.; Murawa, D. Wound fluids affect miR-21, miR-155 and miR-221 expression in breast cancer cell lines, and this effect is partially abrogated by intraoperative radiation therapy treatment. Oncol. Lett. 2017, 14, 4029–4036. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Specification | IORT | Control |
|---|---|---|
| - Histological Subtype | ||
| No special type | 81.0% | 81.0% |
| Lobular histology | 19.0% | 14.3% |
| Tubular histology | 0.0% | 4.8% |
| - Molecular Phenotype | ||
| Luminal A | 57.1% | 61.9% |
| Luminal B (HER2 negative) | 33.3% | 38.1% |
| HER2 positive | 4.8% | 0.0% |
| Triple negative | 4.8% | 0.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuhrer, A.; Uhlig, S.; Tuschy, B.; Berlit, S.; Sperk, E.; Bieback, K.; Sütterlin, M. Wound Fluid from Breast Cancer Patients Undergoing Intraoperative Radiotherapy Exhibits an Altered Cytokine Profile and Impairs Mesenchymal Stromal Cell Function. Cancers 2021, 13, 2140. https://doi.org/10.3390/cancers13092140
Wuhrer A, Uhlig S, Tuschy B, Berlit S, Sperk E, Bieback K, Sütterlin M. Wound Fluid from Breast Cancer Patients Undergoing Intraoperative Radiotherapy Exhibits an Altered Cytokine Profile and Impairs Mesenchymal Stromal Cell Function. Cancers. 2021; 13(9):2140. https://doi.org/10.3390/cancers13092140
Chicago/Turabian StyleWuhrer, Anne, Stefanie Uhlig, Benjamin Tuschy, Sebastian Berlit, Elena Sperk, Karen Bieback, and Marc Sütterlin. 2021. "Wound Fluid from Breast Cancer Patients Undergoing Intraoperative Radiotherapy Exhibits an Altered Cytokine Profile and Impairs Mesenchymal Stromal Cell Function" Cancers 13, no. 9: 2140. https://doi.org/10.3390/cancers13092140