Thermodynamic Theory of Phase Separation in Nonstoichiometric Si Oxide Films Induced by High-Temperature Anneals
Abstract
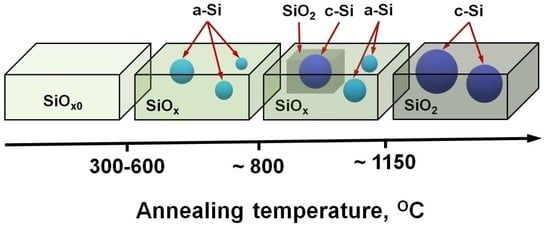
:1. Introduction
2. Theory and Results
2.1. Gibbs Free Energy of Si/Si Oxide Systems
- The Gibbs free energy of the a-Si phase is expressed in terms of the Gibbs free energy of c-Si plus the excess energy equal to per one Si atom [51]. Here, hE = 13,400 J/mole is the molar crystallization enthalpy [52], sE = 3.97 J/mole × K is the molar excess entropy of amorphous-to-crystalline transition [53], and NA = 6.022 × 1023 mole−1 is the Avogadro constant, respectively.
- The nonstoichiometric Si oxide phase is considered in the binary solution approximation [54], in which the SiOx solution is formed by mixing elemental Si and oxygen atoms obtained by the decomposition of O2 molecules.
- The structure of Si oxide is made up of interconnected Si–OySi4–y tetrahedral units (0 ≤ y ≤ 4) with different oxidation degrees y of a central Si atom. In addition to the Si–Si and Si–O bond energies, each tetrahedral unit is characterized by the penalty energy Δy as the measure of the energy nonequivalence of the units with different values of y (Δ0 = Δ4 = 0 eV, Δ1 = 0.5 eV, Δ2 = 0.51 eV, and Δ3 = 0.22 eV) [55]. The probability of finding a tetrahedral unit with either value of y obeys the random bonding model proposed by Phillip [56].
- The entropy of mixing is considered to be the configuration entropy of the Si oxide phase, associated with the number of arrangements of oxygen atoms between the pairs of Si atoms.
2.2. Effect of Nano-Si/Si Oxide Matrix Interfaces on Phase Equilibria in Si/Si Oxide Systems
2.3. Influence of Internal Stress on Phase Separation in SiOx Films
- the superlinear dependence on the difference between the current and the initial Si oxide stoichiometry indexes, to be able to reproduce the increase in the value of xeq with the increase in x0;
- the descending dependence on the annealing temperature, to enable the minimum Gibbs free energy of a Si/Si oxide system shift toward smaller x, upon raising the temperature.
2.4. Crystallization Model of Amorphous Si Nanoinclusions Embedded in the Si Oxide Matrix
- The crystallization of an amorphous Si nanoinclusion is incomplete, and an amorphous shell between the crystalline core and the Si oxide matrix always remains. This result is illustrated by the exemplary dependences of Δgcryst on ξ for different initial radii R at 900 °C presented in Figure 8a, in which ξmin corresponds to the equilibrium states.
- A minimum radius of amorphous Si nanoinclusions exists, below which crystallization becomes energetically unfavorable. The value of this radius increases with the increase in annealing temperature. The dependence of the Gibbs free energy change on ξ for a Si nanoinclusion, with the radius below the crystallization threshold, is illustrated by the curve (1) in Figure 8.
- The crystallized Si fraction (value of ξmin) increases, and the nucleation barrier for crystallization decreases, with the increase in a-Si nanoinclusion size, due to the weakening of the influence of the Si oxide matrix on the crystallization process. The saturation of the nucleation barrier at R ≈ 2 nm points to an almost complete loss of this influence. This result is illustrated by the dependences presented in Figure 9a,b for the crystallization temperature of 900 °C (see also Figure 8a,b).
3. Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Irrera, A.; Franzò, G.; Iacona, F.; Canino, A.; Di Stefano, G.; Sanfilippo, D.; Piana, A.; Fallica, P.D.; Priolo, F. Light emitting devices based on silicon nanostructures. Physica E 2007, 38, 181. [Google Scholar] [CrossRef]
- Wang, M.; Anopchenko, A.; Marconi, A.; Moser, E.; Prezioso, S.; Pavesi, L.; Pucker, G.; Bellutti, P.; Vanzetti, L. Light emitting devices based on nanocrystalline-silicon multilayer structure. Physica E 2009, 41, 912. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Dimitrakis, P.; Kolliopoulou, S.; Normand, P. Recent advances in nanoparticle memories. Mater. Sci. Eng. B 2005, 124–125, 93. [Google Scholar] [CrossRef]
- Bratus’, O.L.; Evtukh, A.A.; Ievtukh, V.A.; Litovchenko, V.G. Nanocomposite SiO2(Si) films as a medium for non-volatile memory. J. Non-Cryst. Solids 2008, 354, 4278. [Google Scholar] [CrossRef]
- Shieh, J.-M.; Lai, Y.-F.; Ni, W.-X.; Kuo, H.-C.; Fang, C.-Y.; Huang, J.Y.; Pan, C.-L. Enhanced photoresponse of a metal-oxide-semiconductor photodetector with silicon nanocrystals embedded in the oxide layer. Appl. Phys. Lett. 2007, 90, 051105. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Aceves-Mijares, M. A ultraviolet-visible-near infrared photodetector using nanocrystalline Si superlattice. Appl. Phys. Lett. 2009, 95, 081101. [Google Scholar] [CrossRef]
- Evtukh, A.A.; Litovchenko, V.G.; Semenenko, M.O. Electrical and emission properties of nanocomposite SiOx(Si) and SiO2(Si) films. J. Vac. Sci. Technol. B 2006, 24, 945. [Google Scholar] [CrossRef]
- Semenenko, M.; Evtukh, A.; Yilmazoglu, O.; Hartnagel, H.L.; Pavlidis, D. A novel method to form conducting channels in SiOx(Si) films for field emission application. J. Appl. Phys. 2010, 107, 013702. [Google Scholar] [CrossRef]
- Yan, B.; Yue, G.; Sivec, L.; Yang, J.; Guha, S.; Jiang, C.-S. Innovative dual function nc-SiOx:H layer leading to a >16% efficient multi-junction thin-film silicon solar cell. Appl. Phys. Lett. 2011, 99, 113512. [Google Scholar] [CrossRef]
- Kale, A.S.; Nemeth, W.; Guthrey, H.; Kennedy, E.; Norman, A.G.; Page, M.; Al-Jassim, M.; Young, D.L.; Agarwal, S.; Stradins, P. Understanding the charge transport mechanisms through ultrathin SiOx layers in passivated contacts for high-efficiency silicon solar cells. Appl. Phys. Lett. 2019, 114, 083902. [Google Scholar] [CrossRef]
- Talbot, E.; Larde’, R.; Pareige, P.; Khomenkova, L.; Hijazi, K.; Gourbilleau, F. Nanoscale evidence of erbium clustering in Er-doped silicon-rich silica. Nanoscale Res. Lett. 2013, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Voitovych, V.V.; Rudenko, R.M.; Yuchymchuk, V.O.; Voitovych, M.V.; Krasko, M.M.; Kolosiuk, A.G.; Povarchuk, V.Y.; Khachevich, I.M.; Rudenko, M.P. Effect of tin on structural transformations in the thin-film silicon suboxide matrix. Ukr. J. Phys. 2016, 61, 980. [Google Scholar] [CrossRef]
- Michailovska, K.V.; Indutnyi, I.Z.; Shepeliavyi, P.E.; Sopinskyy, M.V.; Dan’ko, V.A.; Yukhymchuk, V.O. Luminescent and Raman study of nanostructures formed upon annealing of SiOx:Sm films. Semicond. Phys. Quant. Electr. Optoelectr. 2023, 26, 068. [Google Scholar] [CrossRef]
- Kizjak, A.Y.; Evtukh, A.A.; Bratus, O.L.; Antonin, S.V.; Ievtukh, V.A.; Pylypova, O.V.; Fedotov, A.K. Electron transport through composite SiO2(Si)&FexOy(Fe) thin films containing Si and Fe nanoclusters. J. Alloys Compd. 2022, 903, 163892. [Google Scholar]
- Evtukh, A.; Kizjak, A.; Bratus’, O.; Voitovych, M.; Romanyuk, V.; Mamykin, S.; Antonin, S.; Muriy, Y.; Klymenko, V.; Sarikov, A. Structure and electrical conductivity of nanocomposite SiOxNy(Si) and SiAlzOxNy(Si) films. J. Alloys Compd. 2023, 960, 170879. [Google Scholar] [CrossRef]
- Garrido, B.; Lopez, M.; Perez-Rodrıguez, A.; Garcıa, C.; Pellegrino, P.; Ferre, R.; Moreno, J.A.; Morante, J.R.; Bonafos, C.; Carrada, M.; et al. Optical and electrical properties of Si-nanocrystals ion beam synthesized in SiO2. Nucl. Instr. Methods Phys. Res. B 2004, 216, 213. [Google Scholar] [CrossRef] [Green Version]
- You, L.; Heng, C.L.; Ma, S.Y.; Ma, Z.C.; Zong, W.H.; Wu, Z.; Qin, G.G. Precipitation and crystallization of nanometer Si clusters in annealed Si-rich SiO2 films. J. Cryst. Growth 2000, 212, 109. [Google Scholar] [CrossRef]
- Perego, M.; Fanciulli, M.; Bonafos, C.; Cherkashin, N. Synthesis of mono and bi-layer of Si nanocrystals embedded in a dielectric matrix by e-beam evaporation of SiO/SiO2 thin films. Mater. Sci. Eng. C 2006, 26, 835. [Google Scholar] [CrossRef]
- Haque, S.M.; De, R.; Prathap, C.; Srivastava, S.K.; Rao, K.D. E-beam evaporation of silicon: Native oxidation and quasicontinuous tailoring of optical properties. Phys. Stat. Solidi A 2021, 218, 2100299. [Google Scholar] [CrossRef]
- Laube, J.; Gutsch, S.; Hiller, D.; Bruns, M.; Kübel, C.; Weiss, C.; Zacharias, M. Formation of size controlled silicon nanocrystals in nitrogen free silicon dioxide matrix prepared by plasma enhanced chemical vapor deposition. J. Appl. Phys. 2014, 116, 223501. [Google Scholar] [CrossRef]
- Klingsporn, M.; Kirner, S.; Villringer, C.; Abou-Ras, D.; Costina, I.; Lehmann, M.; Stannowski, B. Resolving the nanostructure of plasma-enhanced chemical vapor deposited nanocrystalline SiOx layers for application in solar cells. J. Appl. Phys. 2016, 119, 223104. [Google Scholar] [CrossRef] [Green Version]
- Tomozeiu, N.; Van Faassen, E.E.; Arnoldbik, W.M.; Vredenberg, A.M.; Habraken, F.H.P.M. Structure of sputtered silicon suboxide single- and multi-layers. Thin Solid Films 2002, 420–421, 382–385. [Google Scholar] [CrossRef]
- Kahler, U.; Hofmeister, H. Visible light emission from Si nanocrystalline composites via reactive evaporation of SiO. Opt. Mater. 2001, 17, 83. [Google Scholar] [CrossRef]
- Sasaki, M.; Ehara, T. Silicon oxide thin films prepared by vacuum evaporation and sputtering using silicon monoxide. J. Phys. Conf. Ser. 2013, 417, 012028. [Google Scholar] [CrossRef]
- Inokuma, T.; Wakayama, Y.; Muramoto, T.; Aoki, R.; Kurata, Y.; Hasegawa, S. Optical properties of Si clusters and Si nanocrystallites in high-temperature annealed SiOx films. J. Appl. Phys. 1998, 83, 2228. [Google Scholar] [CrossRef]
- Wakayama, Y.; Inokuma, T.; Hasegawa, S. Nanoscale structural investigation of Si crystallites grown from silicon suboxide films by thermal annealing. J. Cryst. Growth 1998, 183, 124. [Google Scholar] [CrossRef]
- Comedi, D.; Zalloum, O.H.Y.; Irving, E.A.; Wojcik, J.; Roschuk, T.; Flynn, M.J.; Mascher, P. X-ray-diffraction study of crystalline Si nanocluster formation in annealed silicon-rich silicon oxides. J. Appl. Phys. 2006, 99, 023518. [Google Scholar] [CrossRef] [Green Version]
- Maslova, N.E.; Antonovsky, A.A.; Zhigunov, D.M.; Timoshenko, V.Y.; Glebov, V.N.; Seminogov, V.N. Raman studies of silicon nanocrystals embedded in silicon suboxide layers. Semiconductors 2010, 44, 1040. [Google Scholar] [CrossRef]
- Zacharias, M.; Heitmann, J.; Scholz, R.; Kahler, U.; Bläsing, M.S.J. Size-controlled highly luminescent silicon nanocrystals: A SiO/SiO2 superlattice approach. Appl. Phys. Lett. 2002, 80, 661. [Google Scholar] [CrossRef]
- Hinds, B.J.; Wang, F.; Wolfe, D.M.; Hinkle, C.L.; Lucovsky, G. Investigation of postoxidation thermal treatments of Si/SiO2 interface in relationship to the kinetics of amorphous Si suboxide decomposition. J. Vac. Sci. Technol. B 1998, 16, 2171. [Google Scholar] [CrossRef]
- Dan’ko, V.A.; Indutnyi, I.Z.; Lysenko, V.S.; Maidanchuk, I.Y.; Min’ko, V.I.; Nazarov, A.N.; Tkachenko, A.S.; Shepelyavyi, P.E. Kinetics of structural and phase transformations in thin SiOx films in the course of a rapid thermal annealing. Semiconductors 2005, 39, 1197. [Google Scholar] [CrossRef]
- Zhigunov, D.M.; Seminogov, V.N.; Timoshenko, V.Y.; Sokolov, V.I.; Glebov, V.N.; Malyutin, A.M.; Maslova, N.E.; Shalygina, O.A.; Dyakov, S.A.; Akhmanov, A.S.; et al. Effect of thermal annealing on structure and photoluminescence properties of silicon-rich silicon oxides. Physica E 2009, 41, 1006. [Google Scholar] [CrossRef]
- Wang, M.; Yang, D.; Li, D.; Yuan, Z.; Que, D. Correlation between luminescence and structural evolution of Si-rich silicon oxide film annealed at different temperatures. J. Appl. Phys. 2007, 101, 103504. [Google Scholar] [CrossRef]
- Lisovskyy, I.P.; Voitovich, M.V.; Sarikov, A.V.; Litovchenko, V.G.; Romanyuk, A.B.; Melnik, V.P.; Khatsevich, I.M.; Shepeliavyi, P.E. Transformation of the structure of silicon oxide during the formation of Si nanoinclusions under thermal annealings. Ukr. J. Phys. 2009, 54, 383. [Google Scholar]
- Nagamori, M.; Boivin, J.-A.; Claveau, A. Gibbs free energies of formation of amorphous Si2O3, SiO and Si2O. J. Non-Cryst. Solids 1995, 189, 270. [Google Scholar] [CrossRef]
- Schnurre, S.M.; Gröbner, J.; Schmid-Fetzer, R. Thermodynamics and phase stability in the Si-O system. J. Non-Cryst. Solids 2004, 336, 1. [Google Scholar] [CrossRef]
- La Magna, A.; Nicotra, G.; Bongiorno, C.; Spinella, C.; Grimaldi, M.G.; Rimini, E.; Coffa, L.C.S. Role of the internal strain on the incomplete Si/SiO2 phase separation in substoichiometric silicon oxide films. Appl. Phys. Lett. 2007, 90, 183101. [Google Scholar] [CrossRef]
- Sarikov, A.; Zacharias, M. Gibbs free energy and equilibrium states in the Si/Si oxide systems. J. Phys. Cond. Matter 2012, 24, 385403. [Google Scholar] [CrossRef]
- Sarikov, A. Internal strain mechanism of the incomplete phase separation of nonstoichiometric silicon oxide films. Solid State Commun. 2014, 179, 39. [Google Scholar] [CrossRef]
- Sarikov, A. Mechanisms of the phase separation of nonstoichiometric Si oxide films: What can one learn from thermodynamics? Proc. NAP 2014, 3, 01NTF16. [Google Scholar]
- Lisovskyi, I.P.; Sarikov, A.V.; Sypko, M.I. Thin Film Structures with Silicon Nanoinclusions; Knigi-XXI: Kyiv-Chernivtsi, Ukraine, 2014; Available online: https://www.books-xxi.com.ua/ (accessed on 25 January 2023). (In Ukrainian)
- Sarikov, A. Thermodynamic study of the phase separation mechanisms in nonstoichiometric Si oxide films during high temperature annealing. In Nanomaterials and Nanotechnology; Ahmed, W., Ed.; One Central Press Ltd.: London, UK, 2016; pp. 58–73. [Google Scholar]
- Sarikov, A. Crystallization behavior of amorphous Si nanoinclusions embedded in silicon oxide matrix. Phys. Stat. Solidi A 2020, 217, 1900513. [Google Scholar] [CrossRef]
- Sarikov, A.; Lisovskyy, I. Spinodal decomposition versus nucleation and growth mechanism of phase separation in nonstoichiometric silicon oxide films during high temperature annealing. Solid State Commun. 2019, 287, 19. [Google Scholar] [CrossRef]
- Zelenina, A.; Sarikov, A.; Zhigunov, D.M.; Weiss, C.; Zakharov, N.; Werner, P.; López-Conesa, L.; Estradé, S.; Peiró, F.; Dyakov, S.A.; et al. Silicon nanocrystals in SiNx/SiO2 hetero-superlattices: The loss of size control after thermal annealing. J. Appl. Phys. 2014, 115, 244304. [Google Scholar] [CrossRef]
- Zelenina, A.; Sarikov, A.; Gutsch, S.; Zakharov, N.; Werner, P.; Reichert, A.; Weiss, C.; Zacharias, M. Formation of size-controlled and luminescent Si nanocrystals from SiOxNy/Si3N4 hetero-superlattices. J. Appl. Phys. 2015, 117, 175303. [Google Scholar] [CrossRef]
- Zhigunov, D.M.; Sarikov, A.; Chesnokov, Y.M.; Vasiliev, A.L.; Zakharov, N.; Kashkarov, P.K. Thickness and temperature depending intermixing of SiOx/SiO2 and SiOxNy/SiO2 superlattices: Experimental observation and thermodynamic modeling. Appl. Phys. Lett. 2016, 108, 223102. [Google Scholar] [CrossRef] [Green Version]
- Zhigunov, D.M.; Sarikov, A.; Chesnokov, Y.M.; Vasiliev, A.L.; Zakharov, N.; Kashkarov, P.K. Response to “Comment on ‘Thickness and temperature depending intermixing of SiOx/SiO2 and SiOxNy/SiO2 superlattices: Experimental observation and thermodynamic modelling’”. [Appl. Phys. Lett. 109, 166101 (2016)]. Appl. Phys. Lett. 2016, 109, 166102. [Google Scholar] [CrossRef] [Green Version]
- Sarikov, A.; Zhigunov, D. Thermodynamic mechanism of the intermixing of multilayered structures in the SiOx/SiO2 superlattices with nanometer thick layers. Mater. Today Commun. 2017, 13, 163–169. [Google Scholar] [CrossRef]
- Lisovskyy, I.P.; Voitovych, M.V.; Zlobin, S.O.; Lukianov, A.N.; Oberemok, O.S.; Dubikovsky, O.V.; Sarikov, A.V. Infrared study of the structure of silicon oxynitride films produced by plasma enhanced chemical vapor deposition. J. Non-Cryst. Solids, 2023; under revision. [Google Scholar]
- Nast, O. The Aluminium-Induced Layer Exchange Forming Polycrystalline Silicon on Glass for Thin-Film Solar Cells. Ph.D. Thesis, Philipps-Universität Marburg, Marburg, Germany, 2000. [Google Scholar]
- Donovan, E.P.; Spaepen, F.; Poate, J.M.; Jacobson, D.C. Homogeneous and interfacial heat releases in amorphous silicon. Appl. Phys. Lett. 1989, 55, 1516. [Google Scholar] [CrossRef]
- Spinella, C.; Lombardo, S.; Priolo, F. Crystal grain nucleation in amorphous silicon. J. Appl. Phys. 1998, 84, 5383. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Bongiorno, A.; Pasquarello, A. Validity of the bond-energy picture for the energetics at Si–SiO2 interfaces. Phys. Rev. B 2000, 62, R16326. [Google Scholar] [CrossRef]
- Philipp, H.R. Optical properties of non-crystalline Si, SiO, SiOx and SiO2. J. Phys. Chem. Solids 1971, 32, 1935. [Google Scholar] [CrossRef]
- Winer, K. Defect formation in a-Si:H. Phys. Rev. B 1990, 41, 12150. [Google Scholar] [CrossRef] [PubMed]
- Boninelli, S.; Iacona, F.; Franzò, G.; Bongiorno, C.; Spinella, C.; Priolo, F. Formation, evolution and photoluminescence properties of Si nanoclusters. J. Phys. Cond. Matter 2007, 19, 225003. [Google Scholar] [CrossRef]
- Roussel, M.; Talbot, E.; Pareige, P.; Gourbilleau, F. Influence of the supersaturation on Si diffusion and growth of Si nanoparticles in silicon-rich silica. J. Appl. Phys. 2013, 113, 063519. [Google Scholar] [CrossRef] [Green Version]
- Vanhellemont, J.; Claeys, C. A theoretical study of the critical radius of precipitates and its application to silicon oxide in silicon. J. Appl. Phys. 1987, 62, 3960. [Google Scholar] [CrossRef]
- Borghesi, A.; Pivac, B.; Sassella, A.; Stella, A. Oxygen precipitation in silicon. J. Appl. Phys. 1995, 77, 4169. [Google Scholar] [CrossRef]
- Magomedov, M. Dependence of the surface energy on the size and shape of a nanocrystal. Phys. Solid. State 2004, 46, 954. [Google Scholar] [CrossRef]
- Lu, H.M.; Jiang, Q. Size-dependent surface energies of nanocrystals. J. Phys. Chem. 2004, 108, 5617. [Google Scholar] [CrossRef]
- Zacharias, M.; Streitenberger, P. Crystallization of amorphous superlattices in the limit of ultrathin films with oxide interfaces. Phys. Rev. B 2000, 62, 8391. [Google Scholar] [CrossRef] [Green Version]
- Peibst, R.; Dürkop, T.; Bugiel, E.; Fissel, A.; Costina, I.; Hofmann, K.R. Driving mechanisms for the formation of nanocrystals by annealing of ultrathin Ge layers in SiO2. Phys. Rev. B 2009, 79, 195316. [Google Scholar] [CrossRef]
- Lisovskyy, I.P.; Indutnyy, I.Z.; Gnennyy, B.N.; Lytvyn, P.M.; Mazunov, D.O.; Oberemok, A.S.; Sopinskyy, N.V.; Shepelyavyi, P.E. Structural-phase transformations in SiOx films in the course of vacuum heat treatment. Semiconductors 2003, 37, 97. [Google Scholar] [CrossRef]
- Sarikov, A.; Litovchenko, V.; Lisovskyy, I.; Maidanchuk, I.; Zlobin, S. Role of oxygen migration in the kinetics of the phase separation of nonstoichiometric silicon oxide films during high-temperature annealing. Appl. Phys. Lett. 2007, 91, 133109. [Google Scholar] [CrossRef]
- Sarikov, A. Kinetic model of precipitate growth during phase separation in metastable binary solid solutions. Solid State Phenom. 2016, 242, 196. [Google Scholar] [CrossRef]
- Van Hapert, J.J.; Vredenberg, A.M.; van Faassen, E.E.; Tomozeiu, N.; Arnoldbik, W.M.; Habraken, F.H.P.M. Role of spinodal decomposition in the structure of SiOx. Phys. Rev. B 2004, 69, 245202. [Google Scholar] [CrossRef]
- Pivot, J. Mechanical properties of SiOx thin films. Thin Solid Films 1982, 89, 175. [Google Scholar] [CrossRef]
- Sze, S.M. Physics of Semiconductor Devices, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Wortman, J.J.; Evans, R.A. Young’s modulus, shear modulus, and Poisson’s ratio in silicon and germanium. J. Appl. Phys. 1965, 36, 153. [Google Scholar] [CrossRef]
- Lucovsky, G.; Phillips, J.C. Bond strain and defects at Si–SiO2 and internal dielectric interfaces in high-k gate stacks. J. Phys. Condens. Matter. 2004, 16, S5139. [Google Scholar] [CrossRef]
- Yi, L.X.; Heitmann, J.; Scholz, R.; Zacharias, M. Si rings, Si clusters, and Si nanocrystals—Different states of ultrathin SiOx layers. Appl. Phys. Lett. 2002, 81, 4248. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Liu, Y.; Chen, T. Evolution of Si suboxides into Si nanocrystals during rapid thermal annealing as revealed by XPS and Raman studies. J. Cryst. Growth 2009, 311, 1296. [Google Scholar] [CrossRef]
- Hernández, S.; Martínez, A.; Pellegrino, P.; Lebour, Y.; Garrido, B.; Jordana, E.; Fedeli, J.M. Silicon nanocluster crystallization in SiOx films studied by Raman scattering. J. Appl. Phys. 2008, 104, 144304. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarikov, A. Thermodynamic Theory of Phase Separation in Nonstoichiometric Si Oxide Films Induced by High-Temperature Anneals. Nanomanufacturing 2023, 3, 293-314. https://doi.org/10.3390/nanomanufacturing3030019
Sarikov A. Thermodynamic Theory of Phase Separation in Nonstoichiometric Si Oxide Films Induced by High-Temperature Anneals. Nanomanufacturing. 2023; 3(3):293-314. https://doi.org/10.3390/nanomanufacturing3030019
Chicago/Turabian StyleSarikov, Andrey. 2023. "Thermodynamic Theory of Phase Separation in Nonstoichiometric Si Oxide Films Induced by High-Temperature Anneals" Nanomanufacturing 3, no. 3: 293-314. https://doi.org/10.3390/nanomanufacturing3030019