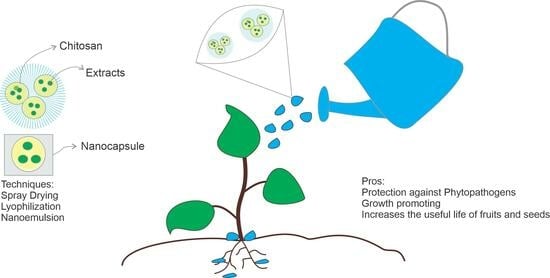
Potential Agricultural Uses of Micro/Nano Encapsulated Chitosan: A Review
Abstract
:1. Introduction
2. Exclusion Criteria
3. Chitosan as Encapsulation Agent
3.1. Chitosan Generalities
3.2. Encapsulations Techniques for Chitosan Micro/Nanoparticles
3.2.1. Spray-Drying
3.2.2. Lyophilization
3.2.3. Nanoemulsions
3.3. Chitosan Micro/Nanoparticles Has Encapsulated Agent of Bioactive Compounds from Plants
3.3.1. Phenolic Compounds
3.3.2. Essential Oils
3.3.3. Others
4. Chitosan Micro/Nanoparticles against Biotic Plant Stress (In Vitro)
4.1. Fungi
4.2. Bacteria
4.3. Nematodes
4.4. Others
5. Chitosan Micro/Nanoparticle Potential on Crops Susceptible to Diseases Caused by Pathogens (In Vivo)
5.1. Cereals
5.2. Fruits
5.3. Vegetables
6. Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riseh, R.S.; Tamanadar, E.; Hajabdollahi, N.; Vatankhah, M.; Thakur, V.K.; Skorik, Y.A. Chitosan microencapsulation of rhizobacteria for biological control of plant pests and diseases: Recent advances and applications. Rhizosphere 2022, 23, 100565. [Google Scholar] [CrossRef]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent advances in extraction of chitin and Chitosan. World J. Microbiol. Biotechnol. 2022, 39, 28. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.D.; Garrido-Miranda, K.A.; Schoebitz, M. Chitin and its derivatives: Functional biopolymers for developing bioproducts for sustainable agriculture—A reality? Carbohydr. Polym. 2023, 299, 120196. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, L.; Khan, I.M.; Yue, L.; Zhang, Y.; Wang, Z. Emerging chitosan grafted essential oil components: A review on synthesis, characterization, and potential application. Carbohydr. Polym. 2022, 297, 120011. [Google Scholar] [CrossRef]
- Kocięcka, J.; Liberacki, D. The Potential of Using Chitosan on Cereal Crops in the Face of Climate Change. Plants 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, L.E.; Popova, E.V.; Novikova, I.I.; Kolesnikova, Y.R.; Balagurova, E.D. Application of Chitosan to Protect Wheat from Diseases and Boost Yields. Appl. Biochem. Microbiol. 2022, 58, 329–335. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Puteh, A.B.; Dafader, N.C.; Malek, M.I.A.; Ehsan, S.D. Foliar application of Chitosan improves growth and yield in maize. J. Food Agric. Environ. 2013, 11, 520–523. [Google Scholar]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of Chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Wan Ahmad Sofian, W.S.Q.; Baharulrazi, N.; Yunus, N.A.; Musa, S.F.M.; Adrus, N.; Jamaludin, J. Foliar application of Chitosan increases plant growth and ecofriendly control of Cucumis sativus leaf disease. Environ. Qual. Manag. 2022, 32, 397–403. [Google Scholar] [CrossRef]
- Maulidna; Wirjosentono, B.; Tamrin; Marpaung, L. Microencapsulation of ginger-based essential oil (Zingiber cassumunar roxb) with Chitosan and oil palm trunk waste fiber prepared by spray-drying method. Case Stud. Therm. Eng. 2020, 18, 100606. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hosseini, S.M.; Hashemi, M. Emerging chitosan nanoparticles loading-system boosted the antibacterial activity of Cinnamomum zeylanicum essential oil. Ind. Crops Prod. 2020, 155, 112824. [Google Scholar] [CrossRef]
- Pecarski, D.; Kneievic-Jugovic, Z.; Dimitrijevic-Brankovic, S.; Mihajilovski, K.; Jankovic, S. Preparation, characterization and antimicrobial activity of chitosan microparticles with thyme essential oil. Hem. Ind. 2014, 68, 721–729. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Cabanillas-Bojórquez, L.A.; Montes-Ávila, J.; Vega-García, M.O.; López-Moreno, H.S.; Castillo-López, R.I.; Gutiérrez-Dorado, R. Effect of Optimized Chitosan Coating Obtained by Lactic Fermentation Chemical Treatment of Shrimp Waste on the Post-Harvest Behavior of Fresh-Cut Papaya (Carica papaya L.). Fermentation 2023, 9, 220. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Sukweenadhi, J.; Sunderraj, S.; Shanmugam, R.; Thangavelu, L.; Mijakovic, I.; Perumalsamy, H. Chitosan, chitosan nanoparticles and modified chitosan biomaterials, a potential tool to combat salinity stress in plants. Carbohydr. Polym. 2022, 284, 119189. [Google Scholar] [CrossRef] [PubMed]
- Betchem, G.; Johnson, N.A.N.; Wang, Y. The application of chitosan in the control of post-harvest diseases: A review. J. Plant Dis. Prot. 2019, 126, 495–507. [Google Scholar] [CrossRef]
- Caro-León, F.J.; López-Martínez, L.M.; Lizardi-Mendoza, J.; Argüelles-Monal, W.; Goycoolea-Valencia, F.M.; Carvajal-Millán, E.; López-Franco, Y.L. Métodos de preparación de nanopartículas de quitosano: Una revisión. Biotecnia 2019, 21, 13–25. [Google Scholar] [CrossRef]
- Gallegos-Morales, G.; Sánchez-Yáñez, J.M.; Hernández-Castillo, F.D. Chitosan in the protection of agricultural crops against phytopathogens agents. Hortic. Int. J. 2022, 6, 168–175. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Millán, M.d.J.; Carrasco-Portugal, M.d.C.; Heredia, J.B.; Bastidas-Bastidas, P.d.J.; Gutiérrez-Grijalva, E.P.; León-Félix, J.; Angulo-Escalante, M.Á. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants 2023, 12, 1692. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray drying for the encapsulation of oils—A review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Langford, A.; Bhatnagar, B.; Walters, R.; Tchessalov, S.; Ohtake, S. Drying technologies for biopharmaceutical applications: Recent developments and future direction. Dry. Technol. 2018, 36, 677–684. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray drying: An overview. In Biomaterials—Physics and Chemistry—New Edition; InTech Open: London, UK, 2018; pp. 9–35. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- do Vale Morais, A.R.; do Nascimento Alencar, É.; Júnior, F.H.X.; De Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; Do Egito, E.S.T.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Gangurde, J.S.; Erande, K.; Shevale, L. Freeze drying: A review. World J. Pharm. Res. 2019, 8, 592–603. [Google Scholar]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225. [Google Scholar] [CrossRef]
- Singh, K.G.a.S.K. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Choradiya, B.R.; Patil, S.B. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef]
- Safaya, M.; Rotliwala, Y.C. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Aljabri, N.M.; Shi, N.; Cavazos, A. Nanoemulsion: An emerging technology for oilfield applications between limitations and potentials. J. Pet. Sci. Eng. 2022, 208, 109306. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Chapter 6—Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 169–188. [Google Scholar]
- Sheth, T.; Seshadri, S.; Prileszky, T.; Helgeson, M.E. Multiple nanoemulsions. Nat. Rev. Mater. 2020, 5, 214–228. [Google Scholar] [CrossRef]
- Gorle, K.A.a.A.P. An overview on methods of preparation and characterization of nanoemulsion. World J. Pharm. Pharm. Sci. 2021, 10, 897–908. [Google Scholar] [CrossRef]
- Borges, D.F.; Lopes, E.A.; Fialho Moraes, A.R.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Moreira Valente, V.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a potential natural compound to manage plant diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef]
- Mazzotta, E.; Muzzalupo, R.; Chiappetta, A.; Muzzalupo, I. Control of the Verticillium Wilt on Tomato Plants by Means of Olive Leaf Extracts Loaded on Chitosan Nanoparticles. Microorganisms 2022, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan Nanoparticles as a Promising Nanomaterial for Encapsulation of Pomegranate (Punica granatum L.) Peel Extract as a Natural Source of Antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Zou, L.-Q.; Liu, W.; Liu, W.-L.; Liang, R.-H.; Li, T.; Liu, C.-M.; Cao, Y.-L.; Niu, J.; Liu, Z. Characterization and Bioavailability of Tea Polyphenol Nanoliposome Prepared by Combining an Ethanol Injection Method with Dynamic High-Pressure Microfluidization. J. Agric. Food Chem. 2014, 62, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Deepika; Prasad, J.; Dwivedy, A.K.; Dubey, N.K. Improvement of in vitro and in situ antifungal, AFB1 inhibitory and antioxidant activity of Origanum majorana L. essential oil through nanoemulsion and recommending as novel food preservative. Food Chem. Toxicol. 2020, 143, 111536. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; Rubio-Moraga, A.; López-Jimenez, A.J.; García Martínez, J.C.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr. Polym. 2022, 277, 118815. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Hesami, G.; Darvishi, S.; Zarei, M.; Hadidi, M. Fabrication of chitosan nanoparticles incorporated with Pistacia atlantica subsp. kurdica ‘hulls’ essential oil as a potential antifungal preservative against strawberry grey mould. Int. J. Food Sci. Technol. 2021, 56, 4215–4223. [Google Scholar] [CrossRef]
- Hadidi, M.; Motamedzadegan, A.; Jelyani, A.Z.; Khashadeh, S. Nanoencapsulation of hyssop essential oil in chitosan-pea protein isolate nano-complex. LWT 2021, 144, 111254. [Google Scholar] [CrossRef]
- Payandeh, M.; Ahmadyousefi, M.; Alizadeh, H.; Zahedifar, M. Chitosan nanocomposite incorporated Satureja kermanica essential oil and extract: Synthesis, characterization and antifungal assay. Int. J. Biol. Macromol. 2022, 221, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Sangsuwan, J.; Sutthasupa, S. Effect of chitosan and alginate beads incorporated with lavender, clove essential oils, and vanillin against Botrytis cinerea and their application in fresh table grapes packaging system. Packag. Technol. Sci. 2019, 32, 595–605. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yilmaz, A.; Akman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Emerg. Technol. 2019, 52, 166–178. [Google Scholar] [CrossRef]
- Amighi, M.; Zahedifar, M.; Alizadeh, H.; Payandeh, M. Encapsulation of Nepeta hormozganica and Nepeta dschuprensis essential oils in shrimp chitosan NPs: Enhanced antifungal activity. Int. J. Biol. Macromol. 2023, 238, 124112. [Google Scholar] [CrossRef]
- Izadi, M.; Moosawi Jorf, S.A.; Nikkhah, M.; Moradi, S. Antifungal activity of hydrocolloid nano encapsulated Carum copticum essential oil and Peganum harmala extract on the pathogenic fungi Alternaria alternata. Physiol. Mol. Plant Pathol. 2021, 116, 101714. [Google Scholar] [CrossRef]
- Tofiño-Rivera, A.P.; Castro-Amaris, G.; Casierra-Posada, F. Effectiveness of Cymbopogon citratus Oil Encapsulated in Chitosan on Colletotrichum gloeosporioides Isolated from Capsicum annuum. Molecules 2020, 25, 4447. [Google Scholar] [CrossRef]
- Deepika; Singh, A.; Chaudhari, A.K.; Das, S.; Dubey, N.K. Zingiber zerumbet L. essential oil-based chitosan nanoemulsion as an efficient green preservative against fungi and aflatoxin B1 contamination. J. Food Sci. 2021, 86, 149–160. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Wang, Q.-S.; Zhu, X.-N.; Jiang, H.-L.; Wang, G.-F.; Cui, Y.-L. Protective effects of alginate–Chitosan microspheres loaded with alkaloids from Coptis chinensis Franch. and Evodia rutaecarpa (Juss.) Benth.(Zuojin Pill) against ethanol-induced acute gastric mucosal injury in rats. Drug Des. Dev. Ther. 2015, 9, 6151. [Google Scholar] [CrossRef]
- Harangozó, J.G.; Wintgens, V.; Miskolczy, Z.; Amiel, C.; Biczók, L. Nanoparticle formation of chitosan induced by 4-sulfonatocalixarenes: Utilization for alkaloid encapsulation. Colloid Polym. Sci. 2016, 294, 1807–1814. [Google Scholar] [CrossRef]
- Bashir, D.J.; Manzoor, S.; Khan, I.A.; Bashir, M.; Agarwal, N.B.; Rastogi, S.; Arora, I.; Samim, M. Nanonization of Magnoflorine-Encapsulated Novel Chitosan–Collagen Nanocapsules for Neurodegenerative Diseases: In Vitro Evaluation. ACS Omega 2022, 7, 6472–6480. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-modified bilosomes nanogel bearing a natural plant alkaloid for safe management of rheumatoid arthritis inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.-S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-coated PLGA nanoparticles loaded with Peganum harmala alkaloids with promising antibacterial and wound healing activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef]
- Roopashree, K.; Naik, D. Saponins: Properties, applications and as insecticides: A review. Biosci. Trends 2019, 8, 1–14. [Google Scholar]
- Bernela, M.; Ahuja, M.; Thakur, R. Enhancement of anti-inflammatory activity of glycyrrhizic acid by encapsulation in chitosan-katira gum nanoparticles. Eur. J. Pharm. Biopharm. 2016, 105, 141–147. [Google Scholar] [CrossRef]
- Kunjumon, R.; Viswanathan, G.; Jayasree, D.V.; Biju, P.G.; Prakash, P.; Sasidharan, B.C.P.; Baby, S. Madecassoside encapsulated in alginate chitosan nanoparticles exerts anti-excitotoxicity effects in pilocarpine-induced seizure. Phytomed. Plus 2021, 1, 100004. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Xie, H.; Zhang, B.; Zhang, B. Enhancing the Antioxidant Ability of Momordica grosvenorii Saponin to Resist Gastrointestinal Stresses via Microcapsules of Sodium Alginate and Chitosan and Its Application in Beverage. Beverages 2022, 8, 70. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Technol. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Kiirika, L.M.; Stahl, F.; Wydra, K. Phenotypic and molecular characterization of resistance induction by single and combined application of Chitosan and silicon in tomato against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 81, 1–12. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Yakoup, A.Y.; Khaled, Y.; Safwat, A.; El-Shibiny, A. The synergistic effect of using bacteriophages and chitosan nanoparticles against pathogenic bacteria as a novel therapeutic approach. Int. J. Biol. Macromol. 2023, 228, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydr. Polym. 2014, 111, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, A.Y.; Albertengo, L.; Rodríguez, M.S.; Debbaudt, A.; Zúñiga, A.; Casalongué, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired Green Synthesis of Chitosan and Zinc Oxide Nanoparticles with Strong Antibacterial Activity against Rice Pathogen Xanthomonas oryzae pv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Eid, A.R.; Badr, M.M.; Marei, G.I.K. Structure and antimicrobial comparison between N-(benzyl) chitosan derivatives and N-(benzyl) chitosan tripolyphosphate nanoparticles against bacteria, fungi, and yeast. Int. J. Biol. Macromol. 2021, 186, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yi, Y.; Yin, Y.; Cai, Z.; Cai, W.; Li, J.; He, G.; Zhang, J. Bacteria-triggered photodynamic nanosystem based on hematoporphyrin-modified Chitosan for sustainable plant disease control. Eur. Polym. J. 2023, 191, 112035. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhou, X.; Wang, Y.; Liang, Y.; Luo, B.; Dai, Y.; Wei, Z.; Li, S.; He, R.; et al. Molecular mechanism of plant elicitor daphnetin-carboxymethyl chitosan nanoparticles against Ralstonia solanacearum by activating plant system resistance. Int. J. Biol. Macromol. 2023, 241, 124580. [Google Scholar] [CrossRef]
- Yang, E.; Lee, J.-W.; Chang, P.-S.; Park, I.-K. Development of chitosan-coated nanoemulsions of two sulfides present in onion (Allium cepa) essential oil and their nematicidal activities against the pine wood nematode, Bursaphelenchus xylophilus. Environ. Sci. Pollut. Res. 2021, 28, 69200–69209. [Google Scholar] [CrossRef] [PubMed]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A. Phytotoxicity of Chitosan and SiO2 Nanoparticles to Seed Germination of Wheat (Triticum aestivum L.) and Barley (Hordeum vulgare L.) Plants. Not. Sci. Biol. 2017, 9, 242–249. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Murugan, I.; Selvaraj, M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in chickpea. Carbohydr. Polym. 2019, 212, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.M.; Elkafafi, S.H.; Zedan, A.M.G.; Dawoud, S.F.M. Effect of Nano Chitosan on Growth, Physiological and Biochemical Parameters of Phaseolus vulgaris under Salt Stress. J. Plant Prod. 2017, 8, 577–585. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A.; Ahmadi, S.B. Evaluation of Chitosan Nanoparticles Effects on Yield and Yield Components of Barley (Hordeum vulgare L.) under Late Season Drought Stress. J. Water Environ. Nanotechnol. 2018, 3, 22–39. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; Nair, S.J.; Jisha, M.S. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019, 124, 1053–1059. [Google Scholar] [CrossRef]
- Mirbolook, A.; Rasouli-Sadaghiani, M.; Sepehr, E.; Lakzian, A.; Hakimi, M. Fortification of bread wheat with iron through soil and foliar application of iron-organic-complexes. J. Plant Nutr. 2021, 44, 1386–1403. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- Khati, P.; Chaudhary, P.; Gangola, S.; Bhatt, P.; Sharma, A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech 2017, 7, 81. [Google Scholar] [CrossRef]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Hu, P.; Jia, J.; Xu, H. A novel water-based chitosan-La pesticide nanocarrier enhancing defense responses in rice (Oryza sativa L.) growth. Carbohydr. Polym. 2018, 199, 437–444. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.; Hasaneen, M.N.; Omar, A. Effect of Foliar Application of Nano Chitosan NPK Fertilizer on the Chemical Composition of Wheat Grains. Egypt. J. Bot. 2018, 58, 87–95. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, e0902. [Google Scholar] [CrossRef]
- Moenne, A.; González, A. Chitosan-, alginate- carrageenan-derived oligosaccharides stimulate defense against biotic and abiotic stresses, and growth in plants: A historical perspective. Carbohydr. Res. 2021, 503, 108298. [Google Scholar] [CrossRef] [PubMed]
- Esyanti, R.R.; Zaskia, H.; Amalia, A.; Nugrahapraja, D.H. Chitosan Nanoparticle-Based Coating as Post-harvest Technology in Banana. J. Phys. Conf. Ser. 2019, 1204, 012109. [Google Scholar] [CrossRef]
- Sahraei Khosh Gardesh, A.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT 2018, 96, 604–611. [Google Scholar] [CrossRef]
- Ejeromedoghene, O.; Oladipo, A.; Oderinde, O.; Okeke, E.S.; Amolegbe, S.A.; Obuotor, T.M.; Akinremi, C.A.; Adewuyi, S.; Fu, G. Facile Green Synthesis of New Chitosan-Metal Nanoparticles as Nano-Agrofungicide For The Preservation of Postharvest Cherry Fruits. ACS Agric. Sci. Technol. 2021, 1, 664–673. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, S.; Xu, Q.; Xu, L.; Zhu, D.; Li, X.; Shui, Y.; Liu, X.; Bi, X. Effect of Chitosan/Nano-TiO2 Composite Coating on the Postharvest Quality of Blueberry Fruit. Coatings 2021, 11, 512. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in Chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Khalil, M.S.; Abd El-Aziz, M.H.; Selim, R.E.-S. Physiological and morphological response of tomato plants to nano-chitosan used against bio-stress induced by root-knot nematode (Meloidogyne incognita) and Tobacco mosaic tobamovirus (TMV). Eur. J. Plant Pathol. 2022, 163, 799–812. [Google Scholar] [CrossRef]
- Oluoch, G.; Nyongesa, M.; Mamati, E.G.; Matiru, V. Thymol and eugenol nanoparticles elicit expression of Ralstonia solanacearum virulence and potato defense genes and are potential bactericides against potato bacterial wilt. Arch. Phytopathol. Plant Prot. 2022, 55, 1136–1157. [Google Scholar] [CrossRef]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, antioxidant and antibacterial activities of chitosan nanoparticles loaded with nettle essential oil. J. Food Meas. Charact. 2021, 15, 1395–1402. [Google Scholar] [CrossRef]


| Advantages | Disadvantages |
|---|---|
|
|
| Advantages | Disadvantages |
|---|---|
|
|
| High Energy Methods | Low Energy Methods |
|---|---|
|
|
| Essential Oils | Particle Type | Encapsulation Method | Encapsulation Conditions | Results | Reference |
|---|---|---|---|---|---|
| Origanum majorana essential oil (OmEO) | Nanoparticle (CH) | Ionotropic gelation | 1% chitosan 85% deacetylation degree 1.03% Tween 80 1:0 to 1:1 CH: OmEO | 37.25 to 88.06% EE 0.5 to 6.73% LC | [60] |
| Garlic essential oil (GEO) | Nanoparticle (CH) | Ionic gelation | 0.2% chitosan 50–190 kDa 75–85% deacetylation degree 1% Tween 80 1:0 to 1:1 CH: GEO | 23.8 to 32.8% EE 5.2 a 19.4% LC | [61] |
| Zataria multiflora essential oil (ZEO) | Nanoparticle (CSNP) | Ionic gelation | 0.3% chitosan 684 kDa 85% deacetylation degree 1:0 to 1:1 CSNP: ZEO | 3.26 to 45.24% EE 5.22 to 9.05% LC | [62] |
| P. atlantica essential oil (PAHEO) | Nanoparticle (CH) | Ionic gelation | 1% chitosan 60–190 kDa 80% deacetylation degree 0.16% Tween 80 1:0 to 1:1.5 CH:PAHEO | 43.3 to 61.5% EE | [63] |
| Hyssop essential oil HEO | Nanoparticle (chitosan-pea protein CHPP) | Nanoprecipitation | 1% chitosan 60–190 kDA 80% deacetylation degree 0.16% Tween 80 1:1 to 5:1 CHPP: HEO | 55.2 to 87.1% EE | [64] |
| Satureja kermanica essential oil (SKEO) | Nanoparticle (CS) | Ionic gelation | 1% chitosan 1:0 to 1:1 CS:SKEO | 45. 18 to 75.88% EE 2.89 to 7.15% LC | [65] |
| Lavender and clove Eos | Microspheres | Ionic gelation | 1% chitosan 1.9% EO 0.2% tween 20 | Clove: 7.62% EE Lavender: 16.48% EE | [66] |
| Origanum vulgare essential oil (OEO) | Nanoparticle (CH) | Electrospraying | 1% chitosan 70 kDa 75–85% deacetylation degree 1:0 to 1:0.5 CH: OEO | 70.1 to 79.6% EE | [67] |
| Nepeta hormozganica and Nepeta dschuprensis essential oils | Nanoparticle (CH) | Co-precipitation | 0.5% chitosan 1.125% Tween 80 1:0 to 1:1.25 CH:EO | 32.73 to 75.91% EE | [68] |
| Carum copticum essential oil (CEO) | Nanoparticle (CH) | Co-precipitation | 1% chitosan 75–85% deacetylation degree 0.1% Tween 80 | 80% EE 14% LC | [69] |
| Cymbopogon citratus essential oil | Minicapsule | Chitosan-agar | 6 mL chitosan 3.6 mL EO | 83% EE | [70] |
| Zingiber zerumbet essential oil (ZEO) | Nanoparticle (CH) | Ionic gelation | 1.5% chitosan 80% deacetylation degree Tween 80 1:0 to 1:1 CH: ZEO | 51.98 to 84.16% EE 0.53 to 2.16% LC | [71] |
| Essential Oil | Nanoparticle | Fungi | Results | References |
|---|---|---|---|---|
| Origanum majorana L. essential oil (OmEO) | Nanoparticle (CH) | A. flavus, A. fumigatus, A. luchuensis, A. niger, P. chrysogenum, P. italicum, C. cladosporioides, F. poae, A. alternata | MIC (ppm): OmEO: 2500 and OmEO-CH: 1000 Aflatoxins (ppm) to 1000 ppm: OmEO: 0 ppm and OmEO-CH: 0.24 ppm | [60] |
| Garlic essential oil (GEO) | Nanoparticle (NPHD) | F. oxysporum A. niger, A. versicolor | MIC (mg/mL): GEO: 7.5, NPHD: 10, and GEO-NPHD: 2.5 GEO: 7.5, NPHD: without effect, and GEO-NPHD: 5 GEO: 7.5, NPHD: without effect, and GEO-NPHD: 5 | [61] |
| Zataria multiflora essential oil (ZEO) | Nanoparticle (CSNP) | B. cinerea | Inhibition (%) to 1500 ppm: ZEO: 55.7, CSNP: 65.15, and ZEO-CSNP: 96.9 | [62] |
| Zingiber zerumbet essential oil (ZEO) | Nanoparticle (CH) | A. flavus | Inhibition (%) to 1000 ppm: ZEO: 66.8 and ZEO-CH: 100 Aflatoxins (ppm) to 800 ppm: ZEO: 2.65 and ZEO-CH: 0 | [62] |
| P. atlantica essential oil (PAHEO) | Nanoparticle (CNP) | B. cinerea | Inhibition (%) to 20 ppm: PAHEO: 74, CNP: 70, and PAHEO-CNP: 100 | [63] |
| Hyssop essential oil HEO | Nanoparticule (chitosan-pea protein CHPP) | B. cinerea | Inhibition (%) to 2 mg/mL: HEO: 70 and HEO-CHPP: 84.3 | [64] |
| Satureja kermanica essential oil (SKEO) | Nanoparticle (CS) | R. solani, A. alternata, B. cinérea, S. sclerotiorum, F. oxysporum | Inhibition (%) (250 ppm) In all fungi SKEO-CS> SKEO > CS KEO-CS: 100 | [65] |
| Lavender and clove EOs | Microspheres | B. cinerea | Inhibition (%) with 1 g de microspheres not dissolved Clove: 6.84 and Lavender: 16.69 | [66] |
| Origanum vulgare essential oil (OEO) | Nanoparticle (CH) | A. alternata | MIC (% w/v): CH: 0.02% and OEO-CH: 0.005% | [67] |
| Nepeta hormozganica and Nepeta dschuprensis essential oils | Nanoparticle (CS) | R. solani, A. alternata B. cinerea, S. sclerotiorum and F. oxysporum | Inhibition (%) to 500 ppm In all fungi and both essential oils EO-CS > EO > CS EO-CS: 100% inhibition in ambos, both essential oils | [68] |
| Carum copticum essential oil (CEO) | Nanoparticle (NCH) | A. alternata | Inhibition (%) to 200 ppm: NCH: 14.01, CEO: 88.43, and CEO-NCH: 94.22 | [69] |
| Cymbopogon citratus essential oil (CCEO) | Minicapsules (CH) | C. gloeosporioides | Inhibition (%): CH: 3.1, CCEO (1156 ppm): 53.9, and CCEO-CH (1370 ppm): 100 Inhibition (%) for 30 d: CCEO: 0 and CCEO-CH: 100 | [70] |
| Encapsulation Chitosan Strategy | Cereal Study | Application Type | Effect | References |
|---|---|---|---|---|
| Nanoemulsion | Wheat (Triticum aestivum L.) | Seed treatment | Promote plant growth and leaves elongation | [92] |
| Nanoemulsion | Barley (Hordeum vulgare) | Seed treatment | Promote plant growth and leaves elongation | [93] |
| Nanoemulsion | Chickpea (Cicer arietinum) | Seed treatment | Enhance germination and promotes a defense system | [94] |
| Ionotropic gelation | Maize (Zea mays) | Foliar application | Enhance germination, and promote plant growth, stem diameter, and root length | [95] |
| Nanoemulsion | Bean (Phaseolus vulgaris) | Seed treatment | Enhance germination and promote plant growth | [96] |
| Nanoemulsion | Barley (Hordeum vulgare cv. Reyhan) | Foliar and soil applications | Increase grain yield and promote plant growth | [97] |
| Ionotropic gelation | Maize (Zea mays) | Seed treatment and foliar application | Promote plant growth and increase grain yield | [98] |
| Nanoemulsion | Rice (Oryza sativa L.) | Seed treatment | Enhance germination | [99] |
| Nanoemulsion | Wheat (Triticum aestivum L.) | Foliar and soil applications | Increase grain yield and promote plant growth | [100] |
| Nanoemulsion | Chickpea (Cicer arietinum) | Seed treatment | Enhance germination | [101] |
| Nanoemulsion | Maize (Zea mays) | Seed treatment | Enhance germination, promote plant growth, and leaves elongation | [102] |
| Nanoemulsion | Rice (Oryza sativa L.) | Seed treatment and soil application | Promote plant growth and increase grain yield | [103] |
| Nanoemulsion | Wheat (Triticum aestivum L.) | Foliar application | Increase grain yield, as well as enhance the potassium and phosphorus content | [104] |
| Nanoemulsion | Wheat (Triticum aestivum L.) | Foliar application | Promote plant growth and increase grain yield | [105] |
| Essential Oil | Nanoparticle | Vegetable | Fungi | Results | References |
|---|---|---|---|---|---|
| Carum copticum essential oil (CEO) and Peganum harmala extract (PE) | Nanoparticle (NCH) | Tomato plant | A. alternata | Severity: NCH: 35.78 ± 4.40%, CEO: 52.35 ± 3.71%, PE: 30.80 ± 2.06%, CEO-NCH: 18.55 ± 2.11% and NPE-CEO: 6.48 ± 3.71% | [69] |
| Cymbopogon citratus essential oil (CCEO) | Minicapsules (CH) | Topito pepper plants | C. gloeosporioides | MIC: 255 µL of CCEO-CH | [70] |
| Cinnamomum zeylanicum essential oil (CEO) | Nanoparticles (CH) | Cucumber | P. drechsleri | Incidence(1.5 g/L): CSNs: 38.66%, CEO: 75.84% and CEO-CSNs: 0% Severity day 9 (1.5 g/L) CSN: 30%, CEO: 74% and CEO-CSNs: 0% Decay day 21 CSN: 44.87% and CEO-CSNs: 26.1% | [82] |
| Mentha piperita essential oil (MEO) | Nanogel chitosan—cinnamic acid (CS-CI) | Tomato | A. flavus | MIC 4 weeks: MEO: 2100 ppm, CS-CI: 1000 ppm and CS-CI-MEO: 500 ppm | [113] |
| Chitosan | Chitosan nanoparticle | Eggplant | Meloidogyne incognita Tobacco mosaic tobamovirus (TMV) | Only nematode: Reduction J2 (Effectiveness): 64.50% Reduction of gall (Effectiveness): 67.87% Nematode + virus Reduction of J2 (Effectiveness): 66.61% Reduction of galls (Effectiveness): 30.71% | [114] |
| Eugenol and thymol | Nanoparticles (CH) | Potato | Ralstonia solanacearum | Severity: 10.3 to 90 ppm | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Carrasco, M.; Valdez-Baro, O.; Cabanillas-Bojórquez, L.A.; Bernal-Millán, M.J.; Rivera-Salas, M.M.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Potential Agricultural Uses of Micro/Nano Encapsulated Chitosan: A Review. Macromol 2023, 3, 614-635. https://doi.org/10.3390/macromol3030034
García-Carrasco M, Valdez-Baro O, Cabanillas-Bojórquez LA, Bernal-Millán MJ, Rivera-Salas MM, Gutiérrez-Grijalva EP, Heredia JB. Potential Agricultural Uses of Micro/Nano Encapsulated Chitosan: A Review. Macromol. 2023; 3(3):614-635. https://doi.org/10.3390/macromol3030034
Chicago/Turabian StyleGarcía-Carrasco, Melissa, Octavio Valdez-Baro, Luis A. Cabanillas-Bojórquez, Manuel J. Bernal-Millán, María M. Rivera-Salas, Erick P. Gutiérrez-Grijalva, and J. Basilio Heredia. 2023. "Potential Agricultural Uses of Micro/Nano Encapsulated Chitosan: A Review" Macromol 3, no. 3: 614-635. https://doi.org/10.3390/macromol3030034