Augmentation and Evaluation of an Olive Oil Based Polyherbal Combination against Diabetic Cardiomyopathy in Experimental Model of Rodents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs, Chemicals and Reagents
2.2. Experimental Animals
2.3. Screening Model
2.4. Experimental Protocol
2.5. Experimental Procedure
2.5.1. Cardiac Collagen Content
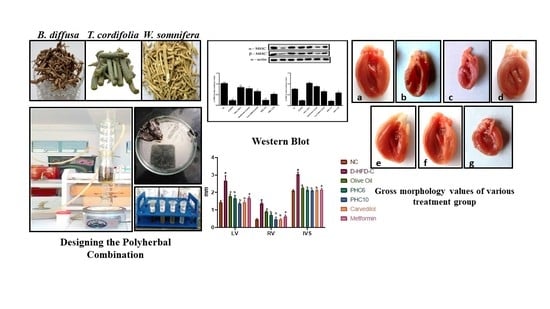
2.5.2. α/β. Myosin Heavy Chain (MHC) Expression (Western Blot)
2.5.3. Troponin-T
2.5.4. Gross Morphological Studies of Whole Heart; Grading of Heart, Ventricle Wall and Intraventricular Septum Thickness
2.5.5. Cardiac Biomarker Enzymes
3. Results
3.1. Initial and Final Blood Glucose Levels
3.2. Food Efficiency Ratio
3.3. Heart Weight/Body Weight Ratio
3.4. Cardiac Biomarker Enzymes
3.5. Estimation of Cardiac Markers
3.6. Cardiac Collagen Content
3.7. α/β. Myosin Heavy Chain (MHC) Expression (Western Blot)
3.8. Troponin-T Test
3.9. Gross Morphological Studies of Whole Heart; Grading of Heart, Ventricle Wall and Intraventricular Septum Thickness
3.10. Histopathological Studies
3.11. Oral Glucose Tolerance Test (OGTT)
4. Discussion
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, C.; Olesen, J.B.; Hansen, P.R.; Weeke, P.; Norgaard, M.L.; Jørgensen, C.H.; Lange, T.; Abildstrøm, S.Z.; Schramm, T.K.; Vaag, A.; et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: A retrospective nationwide cohort study. Diabetologia 2010, 53, 2546–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundbaek, K.; Ledet, T.; Neubauer, B.; Christensen, N.J. Diabetic cardiopathy. Diabetologia 1979, 16, 207–209. [Google Scholar]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Bertoni, A.G.; Tsai, A.; Kasper, E.K.; Brancati, F.L. Diabetes and idiopathic cardiomyopathy: A nationwide case-control study. Diabetes Care 2003, 26, 2791–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, V.; Anandan, E.M.; Mony, R.S.; Muraleedharan, T.S.; Valiathan, M.S.; Mutsuddi, M.; Lakhotia, S.C. In Vivo effects of traditional Ayurvedic formulations in Drosophila melanogaster model relate with therapeutic applications. PLoS ONE 2012, 7, e37113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Kuppurajan, K.; Rajgopalan, S.S.; Sitaram, R.; Rajgopalan, V.; Janaki, K.; Revathi, R.; Vekataraghavan, S. Effects of Ashwagandha on the process of ageing on human volunteers. J. Res. Ayur. Sid. 1989, 1, 247–258. [Google Scholar]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Aphale, A.A.; Chibba, A.D.; Kumbhakarna, N.R.; Mateenuddin, M.O.; Dahat, S.H. Subacute toxicity study of the combination of ginseng (Panax ginseng) and ashwagandha (Withania somnifera) in rats: A safety assessment. Indian J. Physiol. Pharmacol. 1998, 42, 299–302. [Google Scholar]
- Ujowundu, C.O.; Igwe, C.U.; Enemor, V.H.; Nwaogu, L.A.; Okafor, O.E. Nutritive and anti-nutritive properties of Boerhavia diffusa and Commelina nudiflora leaves. Pak. J. Nutr. 2008, 7, 90–92. [Google Scholar] [CrossRef]
- Mungantiwar, A.A.; Nair, A.M.; Kamal, K.K.; Saraf, M.N. Adaptogenic activity of aqueous extract of the roots of Boerhaavia diffusa linn. Indian Drugs 1997, 34, 184–189. [Google Scholar]
- Vermes, E.; Ducharme, A.; Bourassa, M.G.; Lessard, M.; White, M.; Tardif, J.C. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: Insight from the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 2003, 107, 1291–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, J.E.; McGill, J.B.; Santiago, J.V.; Schechtman, K.B.; Waggoner, A.D.; Miller, J.G.; Sobel, B.E. Abnormal myocardial acoustic properties in diabetic patients and their correlation with the severity of disease. J. Am. Coll. Cardiol. 1992, 19, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type II diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Cascio, R.L.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kishore, K.; Sharma, D.; Srinivasan, B.P.; Agarwal, S.S.; Sharma, A.; Singh, S.K.; Gaur, S.; Jatav, V.S. Cardioprotective activity of alcoholic extract of Tinospora cordifolia (Willd.) Miers in calcium chloride-induced cardiac ar-rhythmia in rats. J. Biomed. Res. 2011, 25, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Sumanth, M.; Mustafa, S.S. Antistress, adoptogenic and immunopotentiating activity roots of Boerhaavia diffusa in mice. Int. J. Pharmacol. 2007, 3, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Shamim, A.; Siddiqui, H.H.; Mahmood, T.; Siddiqui, M.H.; Bagga, P.; Ahsan, F.; Shariq, M.; Parveen, S. Pragmatic Toxicity Pro-filing of a Salubrious Polyherbal Combination of Tinospora Cordifolia, Withania Somnifera, and Boerrhavia Diffusa in Swiss Albino Mice. Int. J. Res. Pharm. Sci. 2020, 11, 4240–4252. [Google Scholar] [CrossRef]
- Reed, M.J.; Meszaros, K.; Entes, L.J.; Claypool, M.D.; Pinkett, J.G.; Gadbois, T.M.; Reaven, G.M. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metab. Clin. Exp. 2000, 49, 1390–1394. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Bazoti, F.N.; Bergquist, J.; Markides, K.E.; Tsarbopoulos, A. Noncovalent interaction between amyloid-β-peptide (1–40) and oleuropein studied by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2006, 17, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.T.; Cowan, L.D.; Welty, T.K.; Sievers, M.; Howard, W.J.; Oopik, A.; Wang, W.; Yeh, J.; Devereux, R.B.; Rhoades, E.R.; et al. All-cause morality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988: The Strong Heart Study. Am. J. Epidemiol. 1998, 147, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Tobe, K.; Terauchi, Y.; Eto, K.; Yamauchi, T.; Suzuki, R.; Tsubamoto, Y.; Komeda, K.; Nakano, R.; Miki, H.; et al. Disrup-tion of insulin receptor substrate 2 causes type II diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 2000, 49, 1880–1889. [Google Scholar] [CrossRef] [Green Version]
- Mansor, L.S.; Gonzalez, E.R.; Cole, M.A.; Tyler, D.J.; Beeson, J.H.; Clarke, K.; Carr, C.A.; Heather, L.C. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc. Diabetol. 2013, 12, 136. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.; McNeill, B.; Podrebarac, J.; Hosoyama, K.; Sedlakova, V.; Cron, G.; Smyth, D.; Seymour, R.; Goel, K.; Liang, W.; et al. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocar-dial infarction. Nat. Commun. 2019, 10, 4866. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.; Kumar, S.; Enjamoori, R.; Seth, S.; Dinda, A.K.; Maulik, S.K. Peripheral benzodiazepine receptor ligand Ro5-4864 inhib-its isoprenaline-induced cardiac hypertrophy in rats. Eur. J. Pharmacol. 2010, 644, 146–153. [Google Scholar] [CrossRef]
- Anversa, P.; Sonnenblick, E.H. Ischemic cardiomyopathy: Pathophysiologic mechanisms. Prog. Cardiovasc. Dis. 1990, 33, 49–70. [Google Scholar] [CrossRef]
- Rona, G.; Chappel, C.I.; Kahn, D.S. The significance of factors modifying the development of isoproterenol-induced myocardial necrosis. Am. Heart J. 1963, 66, 389–395. [Google Scholar] [CrossRef]
- Patil, S.; Chaudhary, A.K. Quantitative Estimation of Guduchi Ghana obtained from different amount of water used for Kwa-tha. Int. J. Pharm. Arch. 2013, 2, 160–164. [Google Scholar]











| S. No. | Groups | No. of Animals | Treatment |
|---|---|---|---|
| 1. | Normal Control | 6 | Distilled water 2 mL/kg, p.o. NPD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all the groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of water for injection (WFI) |
| 2. | STZ + HFD-C | 6 | Single i.p. injection of streptozotocin at the dose of 35 mg/kg b.w. (freshly prepared in citrate buffer). HFD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of streptozotocin (35 mg/kg STZ in citrate buffer, pH 4) |
| 3. | Olive Oil | 6 | Single i.p. injection of streptozotocin at the dose of 35 mg/kg b.w. (freshly prepared in citrate buffer). The animals were fed with 2 mL olive oil daily, NPD+ HFD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of streptozotocin (35 mg/kg STZ in citrate buffer, pH 4) |
| 4. | PHC-C6 Treated (Low dose) | 6 | Single i.p. injection of streptozotocin at the dose of 35 mg/kg b.w. (freshly prepared in citrate buffer). The animals were fed with 2 mL PHC-C 6 daily, NPD+ HFD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all the groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of streptozotocin (35 mg/kg STZ in citrate buffer, pH 4) |
| 5. | PHC-C10 Treated (High dose) | 6 | Single i.p. injection of streptozotocin at the dose of 35 mg/kg b.w. (freshly prepared in citrate buffer). The animals were fed with 2 mL PHC-C 10 daily, NPD+ HFD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of streptozotocin (35 mg/kg STZ in citrate buffer, pH 4) |
| 6. | Metformin Treated (70 mg/kg; p.o) | 6 | Single i.p. injection of streptozotocin at the dose of 35 mg/kg b.w. (freshly prepared in citrate buffer). The animals were fed with metformin, NPD + HFD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of streptozotocin (35 mg/kg STZ in citrate buffer, pH 4) |
| 7. | Carvedilol Treated (10 mg/kg; p.o) | 6 | Single i.p. injection of streptozotocin at the dose of 35 mg/kg b.w. (freshly prepared in citrate buffer). The animals were fed with carvedilol, NPD + HFD + water ad libitum for the entire period of study (28 days). On day 13, rodents from all groups including NC were abstained from eating for the time being and given a solitary intraperitoneal infusion of streptozotocin (35 mg/kg STZ in citrate buffer, pH 4) |
| Pipette into Tube Marked Test | Working Reagent Preparation | Remarks | |
|---|---|---|---|
| Serum/Plasma | 100 µL | Added reagent 2 to reagent 1 in 1:4 ratio, i.e., 1 mL of Reagent 2 + 4 mL of Reagent 1. | Non-hemolyzed serum is recommended as RBCs contain ALT activity. For plasma, Heparin or EDTA can be used as anticoagulant. Frequent chilling and thawing of serum results in a rapid loss of ALT activity. |
| Working ALT Reagent | 1000 µL | ||
| Addition Sequence | (T) 25 °C/30 °C | (T) 37 °C | Working Reagent (WR) | Sample Material |
|---|---|---|---|---|
| Pipette the following into a dry and clean test tube labeled as test (T): Sample Working reagent | 0.05 1.0 mL | 0.02 mL 1.0 mL | The working reagent might be made as and when wanted by combining four parts L1 (i.e., buffer reagent) and one part L2 (i.e., starter reagent). On the other hand, 0.8 mL of L1 and 0.2 mL of L2 may likewise be utilized rather than 1 mL of working reagent legitimately during the analysis. | Serum, free from hemolysis, total LDH is described to be steady in serum for 1–3 days at 2–8 °C. Freezing deactivates the liver isoenzyme. |
| Incubate the WR at specific assay temperature for about 1 min and then add. Incubate the mixture at specific assay temperature for about 1 min and mix. | ||||
| Group S. No | NC | D-HFD | Olive Oil-C | Metformin (10 mg/kg) | Carvedilol (2 mg/kg) | PHC-C6 | PHC-C10 |
|---|---|---|---|---|---|---|---|
| 1 | −ve | +ve | +ve | +ve | −ve | +ve | −ve |
| 2 | −ve | +ve | −ve | +ve | −ve | −ve | −ve |
| 3 | −ve | +ve | −ve | +ve | +ve | +ve | −ve |
| 4 | −ve | +ve | −ve | +ve | −ve | −ve | +ve |
| 5 | −ve | +ve | +ve | +ve | −ve | −ve | −ve |
| Groups | Grading of Cardiac Damage |
|---|---|
| NC | Grade 0 |
| D-HFD-C Olive Oil-C | Grade 4 Grade 3 |
| Metformin (70 mg/kg) Carvedilol (2 mg/kg) | Grade 3 Grade 1 |
| PHC6 | Grade 2 |
| PHC10 | Grade 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamim, A.; Siddiqui, H.H.; Mahmood, T.; Wani, T.A.; Zargar, S.; Siddiqui, M.H.; Farooqui, A.; Ahsan, F.; Shariq, M.; Parveen, S.; et al. Augmentation and Evaluation of an Olive Oil Based Polyherbal Combination against Diabetic Cardiomyopathy in Experimental Model of Rodents. Diabetology 2022, 3, 561-582. https://doi.org/10.3390/diabetology3040043
Shamim A, Siddiqui HH, Mahmood T, Wani TA, Zargar S, Siddiqui MH, Farooqui A, Ahsan F, Shariq M, Parveen S, et al. Augmentation and Evaluation of an Olive Oil Based Polyherbal Combination against Diabetic Cardiomyopathy in Experimental Model of Rodents. Diabetology. 2022; 3(4):561-582. https://doi.org/10.3390/diabetology3040043
Chicago/Turabian StyleShamim, Arshiya, Hefazat H. Siddiqui, Tarique Mahmood, Tanveer A. Wani, Seema Zargar, Mohammad Haris Siddiqui, Alvina Farooqui, Farogh Ahsan, Mohammad Shariq, Saba Parveen, and et al. 2022. "Augmentation and Evaluation of an Olive Oil Based Polyherbal Combination against Diabetic Cardiomyopathy in Experimental Model of Rodents" Diabetology 3, no. 4: 561-582. https://doi.org/10.3390/diabetology3040043