4-Hydroxy-2-pyrones: Synthesis, Natural Products, and Application
Abstract
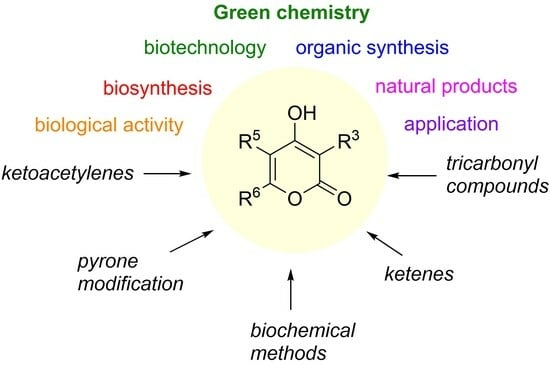
:1. Introduction
2. Synthetic Strategies
2.1. Cyclization of 1,3,5-Tricarbonyl Compounds Derived from Acetoacetic Esters
2.2. Recyclization of Acetals
2.3. Cyclization of Acetylenic 1,3-Dicarbonyl Compounds in the Presence of Gold Complexes
2.4. Syntheses Based on Ketenes
2.5. Pyrone Fragment Modification
3. Natural 4-hydroxy-2-pyrones and Their Applications
4. Biosynthesis and Biotechnological Methods for the Preparation of 4-Hydroxy-2-pyrones
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dobler, D.; Leitner, M.; Moor, N.; Oliver, R. 2-Pyrone—A Privileged Heterocycle and Widespread Motif. Eur. J. Org. Chem. 2021, 2021, 6180–6205. [Google Scholar] [CrossRef]
- Lee, J.S. Recent Advances in the Synthesis of 2-Pyrones. Mar. Drugs 2015, 13, 1581–1620. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S. Pyrone-derived marine natural products: A review on isolation, bio-activities and synthesis. Curr. Org. Chem. 2020, 24, 354–401. [Google Scholar] [CrossRef]
- Ahmad, T.; Rasheed, T.; Hussain, M.; Rizwan, K. Emergence of 2-Pyrone and Its Derivatives, from Synthesis to Biological Perspective: An Overview and Current Status. Top. Curr. Chem. 2021, 379, 38–72. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Lah, H.U.; Yousuf, S.K.; Ahmad, Z. α-pyrones: Small molecules with versatile structural diversity reflected in multiple pharmacological activities-an update. Biomed. Pharmacother. 2017, 91, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schäberle, T.F. Biosynthesis of α-pyrones. Beilstein J. Org. Chem. 2016, 12, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M. Microbial Pyran-2-ones and Dihydropyran-2-ones. Nat. Prod. Rep. 1993, 10, 71–98. [Google Scholar] [CrossRef]
- McGlacken, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef]
- Bhat, Z.S.; Rather, M.A.; Syed, K.Y.; Ahmad, Z. α-Pyrones and their hydroxylated analogs as promising scaffolds against Mycobacterium tuberculosis. Future Med. Chem. 2017, 9, 2053–2067. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, R.; Xia, T.; Zhang, S.; Ma, S.; Guo, Z. Natural Products and Biological Activity from Actinomycetes Associated with Marine Algae. Molecules 2023, 28, 5138. [Google Scholar] [CrossRef]
- Obydennov, D.L.; El-Tantawy, A.I.; Sosnovskikh, V.Y. Triacetic acid lactone as a bioprivileged molecule in organic synthesis. Mendeleev Commun. 2020, 29, 1–10. [Google Scholar] [CrossRef]
- Jilalat, A.E.; Al-Garadi, W.H.A.H.; Karrouchi, K.; Essassi, E.M. Dehydroacetic acid (Part 1): Chemical and pharmacological properties. J. Mar. Chim. Heterocycl. 2017, 16, 1–47. [Google Scholar]
- Fadda, A.A.; Elattar, K.M. Reactivity of Dehydroacetic Acid in Organic Synthesis. Synth. Commun. 2016, 46, 1–30. [Google Scholar] [CrossRef]
- Chen, B.; Xie, Z.; Peng, F.; Li, S.; Yang, J.; Wu, T.; Fan, H.; Zhang, Z.; Hou, M.; Li, S.; et al. Production of Piperidine and δ-Lactam Chemicals from Biomass-Derived Triacetic Acid Lactone. Angew. Chem. Int. Ed. 2021, 60, 14405–14409. [Google Scholar] [CrossRef]
- Demarteau, J.; Cousineau, B.; Wang, Z.; Bose, B.; Cheong, S.; Lan, G.; Baral, N.R.; Teat, S.J.; Scown, C.D.; Keasling, J.D.; et al. Biorenewable and circular polydiketoenamine plastics. Nat. Sustain. 2023, 6, 1426–1435. [Google Scholar] [CrossRef]
- Sajjad, H.; Prebihalo, E.A.; Tolman, W.B.; Reineke, T.M. Ring opening polymerization of β-acetoxy-δ-methylvalerolactone, a triacetic acid lactone derivative. Polym. Chem. 2021, 12, 6724–6730. [Google Scholar] [CrossRef]
- Ortin, G.G.D.; Salles, A.G. Persulfate-promoted synthesis of biphenyl compounds in water from biomass-derived triacetic acid lactone. Org. Biomol. Chem. 2022, 20, 9292–9297. [Google Scholar] [CrossRef]
- Obydennov, D.L.; El-Tantawy, A.I.; Sosnovskikh, V.Y. Bio-based triacetic acid lactone in the synthesis of azaheterocycles via a ring-opening transformation. New J. Chem. 2018, 42, 8943–8952. [Google Scholar] [CrossRef]
- Kornev, M.Y.; Tishin, D.S.; Obydennov, D.L.; Sosnovskikh, V.Y. Reactions of 3-functionalized chromones with triacetic acid lactone. Mendeleev Comm. 2020, 30, 233–235. [Google Scholar] [CrossRef]
- Obydennov, D.L.; El-Tantawy, A.I.; Sosnovskikh, V.Y. Synthesis of Multifunctionalized 2,3-Dihydro-4-pyridones and 4-Pyridones via the Reaction of Carbamoylated Enaminones with Aldehydes. J. Org. Chem. 2018, 83, 13776–13786. [Google Scholar] [CrossRef]
- Huo, J.; Bradley, W.; Podolak, K.; Ryan, B.; Roling, L.T.; Kraus, G.A.; Shanks, B.H. Triacetic Acid Lactone and 4-Hydroxycoumarin as Bioprivileged Molecules for the Development of Performance-Advantaged Organic Corrosion Inhibitors. ACS Sustain. Chem. Eng. 2022, 10, 11544–11554. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Y.; Xu, P.; Deng, L.; Liu, H.; Wang, F. Recent advances and perspectives on the biomass-derived production of the platform chemical triacetic acid lactone by engineered cell factories. Biochem. Eng. J. 2023, 197, 108961. [Google Scholar] [CrossRef]
- Chia, M.; Schwartz, T.J.; Shanks, B.H.; Dumesic, J.A. Triacetic acid lactone as a potential biorenewable platform chemical. Green Chem. 2012, 14, 1850–1853. [Google Scholar] [CrossRef]
- Onda, K.; Hayakawa, I.; Sakakura, A. Reinvestigation of the Biomimetic Cyclization of 3,5-Diketo Esters: Application to the Total Synthesis of Cyercene A, an α-Methoxy-γ-Pyrone-Containing Polypropionate. Synlett 2017, 28, 1596–1600. [Google Scholar]
- Garey, D.; Ramirez, M.-L.; Gonzales, S.; Wertsching, A.; Tith, S.; Keefe, K.; Peña, M.R. An Approach to Substituted 4-Hydroxypyran-2-ones: The Total Synthesis of Phenoxan. J. Org. Chem. 1996, 61, 4853–4856. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, H.; Sunazuka, T.; Izuhara, T.; Hirose, T.; Shiomi, K.; Ōmura, S. Total Synthesis and Biological Evaluation of Verticipyrone and Analogues. Org. Lett. 2007, 9, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Ohba, S.; Nishiyama, S.; Yamamura, S. Total synthesis of phenoxan and a related pyrone derivative. Tetrahedron Lett. 1996, 37, 2997–3000. [Google Scholar] [CrossRef]
- Sharma, P.; Powell, K.J.; Burnley, J.; Awaad, A.S.; Moses, J.E. Total Synthesis of Polypropionate-Derived γ-Pyrone Natural Products. Synthesis 2011, 18, 2865–2892. [Google Scholar]
- Burkhardt, I.; Dickschat, J.S. Synthesis and Absolute Configuration of Natural 2-Pyrones. Eur. J. Org. Chem. 2018, 2018, 3144–3157. [Google Scholar] [CrossRef]
- Rodriguez, R.; Adlington, R.M.; Eade, S.J.; Walter, M.W.; Baldwin, J.E.; Moses, J.E. Total synthesis of cyercene A and the biomimetic synthesis of (±)-9,10-deoxytridachione and (±)-ocellapyrone A. Tetrahedron 2007, 63, 4500–4509. [Google Scholar] [CrossRef]
- Liang, G.; Miller, A.K.; Trauner, D. Stereoselective Synthesis of Cyercene A and the Placidenes. Org. Lett. 2005, 7, 819–821. [Google Scholar] [CrossRef]
- Ramesh, P.; Meshram, H.M. First total synthesis of salinipyrone A using highly stereoselective vinylogous Mukaiyama aldol reaction. Tetrahedron 2012, 68, 9289–9292. [Google Scholar] [CrossRef]
- Shishido, K.; Yoshida, M.; Takai, H.; Mitsuhashi, C. Concise and Efficient Synthesis of 4-Hydroxy-2-pyrones from Pentane-2,4-diones. Heterocycles 2010, 82, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Köster, G.; Hoffmann, R.W. Functionalization of 3,5,6-trialkyl-4-hydroxy-2-pyrones in the 6-α position. Liebigs Ann. Chem. 1987, 1987, 987–990. [Google Scholar] [CrossRef]
- Minassi, A.; Cicione, L.; Koeberle, A.; Bauer, J.; Laufer, S.; Werz, O.; Appendino, G. A Multicomponent Carba-Betti Strategy to Alkylidene Heterodimers—Total Synthesis and Structure–Activity Relationships of Arzanol. Eur. J. Org. Chem. 2012, 2012, 772–779. [Google Scholar] [CrossRef]
- Zhang, F.; Danishefsky, S.J. An Efficient Stereoselective Total Synthesis of dl-Sesquicillin, a Glucocorticoid Antagonist. Ang. Chem. Int. Ed. 2002, 41, 1434–1437. [Google Scholar] [CrossRef]
- Katoh, T. Total Synthesis of Diterpenoid Pyrones, Nalanthalide, Sesquicillin, Candelalides A–C, and Subglutinols A, B. Stud. Nat. Prod. Chem. 2014, 43, 1–39. [Google Scholar]
- Arndt, F. Dehydroacetic acid. Org. Synth. 1940, 20, 26. [Google Scholar]
- Sarhan, A.A.M.; Haukka, M.; Barakat, A.; Boraei, A.T.A. A novel synthetic approach to pyran-2,4-dione scaffold production: Microwave-assisted dimerization, cyclization, and expeditious regioselective conversion into β-enamino-pyran-2,4-diones. Tetrahedron Lett. 2020, 61, 152660. [Google Scholar] [CrossRef]
- Bouthillette, L.M.; Darcey, C.A.; Handy, T.E.; Seaton, S.C.; Wolfe, A.L. Isolation of the antibiotic pseudopyronine B and SAR evaluation of C3/C6 alkyl analogs. Bioorganic Med. Chem. Lett. 2017, 27, 2762–2765. [Google Scholar] [CrossRef]
- Dhage, G.R.; Thopate, S.R.; Ramteke, S.N.; Kulkarni, P.P. One-pot synthesis and evaluation of novel 3-aryl-6-ethoxycarbonyl-4-hydroxy-2H-pyran-2-one as a potent cytotoxic agent. RSC Adv. 2014, 4, 56870–56875. [Google Scholar] [CrossRef]
- Schmidt, D.; Conrad, J.; Klaiber, I.; Beifuss, U. Synthesis of the bis-potassium salts of 5-hydroxy-3-oxopent-4-enoic acids and their use for the efficient preparation of 4-hydroxy-2H-pyran-2-ones and other heterocycles. Chem. Commun. 2006, 45, 4732–4734. [Google Scholar] [CrossRef]
- Lokot, I.P.; Pashkovsky, F.S.; Lakhvich, F.A. A new approach to the synthesis of 3,6- and 5,6-dialkyl derivatives of 4-hydroxy-2-pyrone. Synthesis of rac-germicidin. Tetrahedron 1999, 55, 4783–4792. [Google Scholar] [CrossRef]
- Marsico, G.; Ciccone, M.S.; Masi, M.; Freda, F.; Cristofaro, M.; Evidente, A.; Superchi, S.; Scafato, P. Synthesis and Herbicidal Activity Against Buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules 2020, 24, 3193. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Wang, Z.; Wang, M.; Hall, C.D.; Suzuki, K. Facile Syntheses of 2,2-Dimethyl-6-(2-oxoalkyl)-1,3-dioxin-4-ones and the Corresponding 6-Substituted 4-Hydroxy-2-pyrones. J. Org. Chem. 2005, 70, 4854–4856. [Google Scholar] [CrossRef] [PubMed]
- Basset, J.-F.; Leslie, C.; Hamprecht, D.; White, A.J.P.; Barrett, A.G.M. Studies on the resorcylates: Biomimetic total syntheses of (+)-montagnetol and (+)-erythrin. Tetrahedron Lett. 2010, 51, 783–785. [Google Scholar] [CrossRef]
- Reber, K.P.; Burdge, H.E. Total Synthesis of Pyrophen and Campyrones A–C. J. Nat. Prod. 2018, 81, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Chaładaj, W.; Corbet, M.; Fürstner, A. Total Synthesis of Neurymenolide A Based on a Gold-Catalyzed Synthesis of 4-Hydroxy-2-pyrones. Ang. Chem. Int. Ed. 2012, 51, 6929–6933. [Google Scholar] [CrossRef]
- Fürstner, A. Gold Catalysis for Heterocyclic Chemistry: A Representative Case Study on Pyrone Natural Products. Ang. Chem. Int. Ed. 2018, 57, 4215–4233. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Li, X.; Wang, J.; Yang, Y. Gold(I)-Catalyzed Intermolecular Rearrangement Reaction of Glycosyl Alkynoic β-Ketoesters for the Synthesis of 4-O-Glycosylated 2-Pyrones. J. Org. Chem. 2019, 84, 14141–14150. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, J.; Shin, H.J.; Lee, H.-S.; Lee, Y.-J.; Lee, H.-S.; Won, H. Total Synthesis and Configurational Validation of (+)-Violapyrone C. Eur. J. Org. Chem. 2014, 2014, 4472–4476. [Google Scholar] [CrossRef]
- Luo, T.; Dai, M.; Zheng, S.-L.; Schreiber, S.L. Syntheses of α-Pyrones Using Gold-Catalyzed Coupling Reactions. Org. Lett. 2011, 13, 2834–2836. [Google Scholar] [CrossRef]
- Gayyur; Choudhary, S.; Saxena, A.; Ghosh, N. Gold-catalyzed homo- and cross-annulation of alkynyl carboxylic acids: A facile access to substituted 4-hydroxy 2H-pyrones and total synthesis of pseudopyronine A. Org. Biomol. Chem. 2020, 18, 8716–8723. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Hiranuma, S.; Watanabe, T. Reduction of α-Pyrone Derivatives with Borane-Methyl Sulfide Complex. Heterocycles 1993, 36, 2445–2450. [Google Scholar] [CrossRef]
- Sheibani, H.; Islami, M.R.; Khabazzadeh, H.; Saidi, K. A convenient one-pot synthesis of substituted 2-pyrone derivatives. Tetrahedron 2004, 60, 5931–5934. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Abaszadeh, M.; Saidi, K.; Pourkhosravani, M. A one-pot reaction of (chlorocarbonyl) phenyl ketene with β-ketoamides and thiochromen-2-one. J. Iran. Chem. Soc. 2013, 10, 237–241. [Google Scholar] [CrossRef]
- German, L.S.; Sterlin, S.R.; Cherstkov, V.F. Hexafluorodehydroacetic acid. Bull. Acad. Sci. USSR Div. Chem. Sci. 1982, 31, 1476–1477. [Google Scholar] [CrossRef]
- Fedin, V.V.; Usachev, S.A.; Obydennov, D.L.; Sosnovskikh, V.Y. Reactions of Trifluorotriacetic Acid Lactone and Hexafluorodehydroacetic Acid with Amines: Synthesis of Trifluoromethylated 4-Pyridones and Aminoenones. Molecules 2022, 27, 7098. [Google Scholar] [CrossRef]
- Perrone, S.; Caroli, A.; Cannazza, G.; Granito, C.; Salomone, A.; Troisi, L. A direct synthesis of 3-acyl-4-hydroxy-2-pyranone derivatives via palladium-catalyzed carbonylation of α-chloroketones. A cascade reaction involving acylketenes. Tetrahedron Lett. 2015, 56, 2773–2776. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Castro-Agudelo, B.; Gálvez, J.; Carissan, Y.; Rodríguez, J.; Coquerel, Y. Periselectivity in the aza-Diels–Alder Cycloaddition between α-Oxoketenes and N-(5-Pyrazolyl)imines: A Combined Experimental and Theoretical Study. J. Org. Chem. 2020, 85, 7368–7377. [Google Scholar] [CrossRef]
- Hosokawa, S.; Yokota, K.; Imamura, K.; Suzuki, Y.; Kawarasaki, M.; Tatsuta, K. The first total synthesis and structural determination of actinopyrone A. Tetrahedron Lett. 2006, 47, 5415–5418. [Google Scholar] [CrossRef]
- Usachev, B.I.; Obydennov, D.L.; Röschenthaler, G.-V.; Sosnovskikh, V.Y. 2-Cyano-6-(trifluoromethyl)-4H-pyran-4-one: A novel versatile CF3-containing building block. J. Fluor. Chem. 2012, 137, 22–26. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Suslova, A.I.; Sosnovskikh, V.Y. Synthesis and some chemical properties of 2-cyano-4-pyrone. Chem. Heterocycl. Compd. 2020, 56, 173–179. [Google Scholar] [CrossRef]
- Lu, H.; Handore, K.L.; Wood, T.E.; Shimokura, G.K.; Schimmer, A.D.; Batey, R.A. Total Synthesis of the 2,5-Disubstituted γ-Pyrone E1 UAE Inhibitor Himeic Acid A. Org. Lett. 2023, 25, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Obydennov, D.L.; Röschenthaler, G.-V.; Sosnovskikh, V.Y. An improved synthesis and some reactions of diethyl 4-oxo-4H-pyran-2,5-dicarboxylate. Tetrahedron Lett. 2013, 54, 6545–6548. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Samanta, S.K. Synthesis of Pyrimidine-Annelated Heterocycles: Regioselective Heterocyclization of 5-(Cyclohex-2-enyl)-1,3-dimethyl-6-hydroxyuracil. Monatsh. Chem. 2022, 133, 1187–1192. [Google Scholar] [CrossRef]
- Lei, K.; Li, P.; Yang, X.-F.; Wang, S.-B.; Wang, X.-K.; Hua, X.-W.; Sun, B.; Ji, L.-S.; Xu, X.-H. Design and Synthesis of Novel 4-Hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one Derivatives for Use as Herbicides and Evaluation of Their Mode of Action. J. Agric. Food Chem. 2019, 67, 10489–10497. [Google Scholar] [CrossRef]
- Sharma, A. Dehydroacetic Acid and its Derivatives. In Useful Synthons in Organic Synthesis, 1st ed.; Penta, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 81–118. [Google Scholar]
- Guan, Q.; Lin, C.; Chen, S.; Gao, F.; Shen, L. Palladium-Catalyzed Selective Carbofunctionalization of Inert γ-C(sp3)–O Bonds with 4-Hydroxypyridin-2(1H)-ones and 4-Hydroxy-2H-pyran-2-ones. Adv. Synth. Catal. 2020, 362, 5772–5776. [Google Scholar] [CrossRef]
- Storm, P.A.; Pal, P.; Huitt-Roehl, C.R.; Townsend, C.A. Exploring fungal polyketide C-methylation through combinatorial domain swaps. ACS Chem. Biol. 2018, 13, 3043–3048. [Google Scholar] [CrossRef]
- Ohyoshi, T.; Mitsugi, K.; Ichimura, F.; Higuma, T.; Yoshida, M.; Kigoshi, H. Total Synthesis and Structure-Activity Relationship Studies of Phelligridins C and D, and Phellifur opyranone A. Bull. Chem. Soc. Jpn. 2020, 93, 1540–1551. [Google Scholar] [CrossRef]
- Kikuchi, H.; Hoshi, T.; Kitayama, M.; Sekiya, M.; Katou, Y.; Ueda, K.; Kubohara, Y.; Sato, H.; Shimazu, M.; Kurata, S.; et al. New diterpene pyrone-type compounds, metarhizins A and B, isolated from entomopathogenic fungus, Metarhizium flavoviride and their inhibitory effects on cellular proliferation. Tetrahedron 2009, 65, 469–477. [Google Scholar] [CrossRef]
- Fang, Z.; Liao, P.-C.; Yang, Y.-L.; Yang, F.-L.; Chen, Y.-L.; Lam, Y.; Hua, K.-F.; Wu, S.-H. Synthesis and Biological Evaluation of Polyenylpyrrole Derivatives as Anticancer Agents Acting through Caspases-Dependent Apoptosis. J. Med. Chem. 2010, 53, 7967–7978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, Y.; Bao, R.; Luo, T.; Yang, Z.; Tang, Y. Enantioselective Biomimetic Total Syntheses of Katsumadain and Katsumadain C. Org. Lett. 2012, 14, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.P.; Go, M.K.; Yew, W.S. Exploiting the Biosynthetic Potential of Type III Polyketide Synthases. Molecules 2016, 21, 806. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Clomburg, J.M.; Cheong, S.; Qian, S.; Gonzalez, R. A polyketoacyl-CoA thiolase-dependent pathway for the synthesis of polyketide backbones. Nat. Catal. 2020, 3, 593–603. [Google Scholar] [CrossRef]
- Bentley, R.; Zwitkowits, P.M. Biosynthesis of Tropolones in Penicillium stipitatum. VII.1,2 The Formation of Polyketide Lactones and Other Nontropolone Compounds as a Result of Ethionine Inhibition. J. Am. Chem. Soc. 1967, 89, 676–680. [Google Scholar]
- Igarashi, Y. Development of a drug discovery approach from microbes with a special focus on isolation sources and taxonomy. J. Antibiot. 2023, 76, 365–383. [Google Scholar] [CrossRef]
- Palkina, K.A.; Ipatova, D.A.; Shakhova, E.S.; Balakireva, A.V.; Markina, N.M. Therapeutic potential of hispidin—Fungal and plant polyketide. J. Fungi 2021, 7, 323. [Google Scholar] [CrossRef]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Zhang, W.R.; Ng, S.; Cooper, P.A. Ability of three yellow pigment producing fungi to colour wood under controlled conditions. Int. Wood Prod. J. 2014, 5, 103–107. [Google Scholar] [CrossRef]
- Purtov, K.V.; Petushkov, V.N.; Baranov, M.S.; Mineev, K.S.; Rodionova, N.S.; Kaskova, Z.M.; Tsarkova, A.S.; Petunin, A.I.; Bondar, V.S.; Rodicheva, E.K.; et al. The chemical basis of fungal bioluminescence. Angew. Chem. Int. Ed. 2015, 54, 8124–8128. [Google Scholar] [CrossRef]
- Han, J.-J.; Bao, L.; He, L.-W.; Zhang, X.-Q.; Yang, X.-L.; Li, S.-J.; Yao, Y.-J.; Liu, H.-w. Phaeolschidins A–E, Five Hispidin Derivatives with Antioxidant Activity from the Fruiting Body of Phaeolus schweinitzii Collected in the Tibetan Plateau. J. Nat. Prod. 2013, 76, 1448–1453. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, J.; Wang, L.; Wang, Y.; Kong, F.; Zhu, W. New α-glucosidase inhibitors from marine algae-derived Streptomyces sp. OUCMDZ-3434. Sci. Rep. 2016, 6, 20004–20012. [Google Scholar] [CrossRef]
- Graziani, E.I.; Ritacco, F.V.; Bernan, V.S.; Telliez, J.-B. Phaeochromycins, A-E, Anti-inflammatory Polyketides Isolated from the Soil Actinomycete Streptomyces phaeochromogenes LL-P018. J. Nat. Prod. 2005, 68, 1262–1265. [Google Scholar] [CrossRef]
- Ma, M.; Rateb, M.E.; Yang, D.; Rudolf, J.D.; Zhu, X.; Huang, Y.; Zhao, L.-X.; Jiang, Y.; Duan, Y.; Shen, B. Germicidins H–J from Streptomyces sp. CB00361. J. Antibiot. 2017, 70, 200–203. [Google Scholar] [CrossRef]
- Petersen, F.; Zähner, H.; Metzger, J.W.; Freund, S.; Hummel, R.P. Germicidin, an autoregulative germination inhibitor of Streptomyces viridochromogenes NRRL B-1551. J. Antibiot. 1993, 46, 1126–1138. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Cao, Y.; Liu, J.; Zheng, D.; Chen, X.; Han, L.; Jiang, C.; Huang, X. Violapyrones A–G, α-Pyrone Derivatives from Streptomyces violascens Isolated from Hylobates hoolock Feces. J. Nat. Prod. 2013, 76, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, A.O.; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H.B.; Heermann, R. Pyrones as bacterial signaling molecules. Nat. Chem. Biol. 2013, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.S.; Ghequire, M.G.K.; Nett, M.; Josten, M.; Sahl, H.-G.; De Mot, R.; Gross, H. Biosynthetic Origin of the Antibiotic Pseudopyronines A and B in Pseudomonas putida BW11M1. ChemBioChem 2015, 16, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Xiao, Y.; Sulaiman, J.E.; Luo, F.; Tang, J.-W.; Huang, Y.; Wu, C.; Wu, L.; Wong, W.; Chen, F.; et al. Mechanistic Insight into the Inhibitory Activity of Elasnin-Based Coating against Early Marine Biofilms. Environ. Sci. Technol. 2023, 57, 9515–9525. [Google Scholar] [CrossRef]
- Motuhi, S.-E.; Feizbakhsh, O.; Foll-Josselin, B.; Baratte, B.; Delehouzé, C.; Cousseau, A.; Fant, X.; Bulinski, J.C.; Payri, C.E.; Ruchaud, S.; et al. Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides. Mar. Drugs 2019, 17, 93. [Google Scholar] [CrossRef]
- Hohmann, C.; Schneider, K.; Bruntner, C.; Brown, R.; Jones, A.; Goodfellow, M.; Krämer, M.; Imhoff, J.; Nicholson, G.; Fiedler, H.-P.; et al. Albidopyrone. A new α-pyrone-containing metabolite from marine-derived Streptomyces sp. NTK 227. J. Antibiot. 2009, 62, 75–79. [Google Scholar] [CrossRef]
- Raju, R.; Piggott, A.M.; Quezada, M.; Capon, R.J. Nocardiopsins C and D and nocardiopyrone A: New polyketides from an Australian marine-derived Nocardiopsis sp. Tetrahedron 2013, 69, 692–698. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Zhang, W. Natural Polypropionates in 1999–2020: An Overview of Chemical and Biological Diversity. Mar. Drugs 2020, 18, 569. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Moles, J.; Avila, C.; Fontana, A. Exiguapyrone and exiguaone, new polypropionates from the Mediterranean cephalaspidean mollusc Haminoea exigua. Tetrahedron Lett. 2016, 57, 71–74. [Google Scholar] [CrossRef]
- Ding, L.; Ren, L.; Li, S.; Song, J.; Han, Z.; He, S.; Xu, S. Production of New Antibacterial 4-Hydroxy-α-Pyrones by a Marine Fungus Aspergillus niger Cultivated in Solid Medium. Mar. Drugs 2019, 17, 344. [Google Scholar] [CrossRef]
- Awakawa, T.; Crüsemann, M.; Munguia, J.; Ziemert, N.; Nizet, V.; Fenical, W.; Moore, B.S. Salinipyrone and Pacificanone Are Biosynthetic Byproducts of the Rosamicin Polyketide Synthase. ChemBioChem 2015, 16, 1443–1447. [Google Scholar] [CrossRef]
- Xian, P.-J.; Chen, H.-Y.; Feng, Z.; Zhao, W.; Yang, X.-L. Capsulactone: A new 4-hydroxy-α-pyrone derivative from an endophytic fungus Penicillium capsulatum and its antimicrobial activity. J. Asian. Nat. Prod. Res. 2021, 23, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Gregg, C.; Perkins, M.V. Total synthesis and structural elucidation of ent-micropyrone and (+)-ascosalipyrone. Org. Biomol. Chem. 2012, 10, 6453–6547. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takahashi, K.; Iwakura, N.; Abe, T.; Akaishi, M.; Chiba, S.; Namikoshi, M.; Uchida, R. A new protein tyrosine phosphatase 1B inhibitory α-pyrone-type polyketide from Okinawan plant-associated Aspergillus sp. TMPU1623. J. Antibiot. 2018, 71, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-Y.; He, J.-J.; Su, J.-Q.; Kong, S.-Z.; Su, J.-Y.; Li, Y.-C.; Huang, S.-H.; Li, C.-W.; Lai, X.-P.; Su, Z.-R. Progostone Synthesis and antimicrobial evaluation of pogostone and its analogues. Fitoterapia 2013, 84, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Xian, J.-D.; Kong, S.-Z.; Li, Y.-C.; Xie, J.-H.; Lin, J.; Chen, J.-N.; Wang, H.-F.; Su, Z.-R. Insecticidal activity of pogostone against Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae). Pest. Manag. Sci. 2014, 70, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Seshime, Y.; Juvvadi, P.R.; Kitamoto, K.; Ebizuka, Y.; Fujii, I. Identification of csypyrone B1 as the novel product of Aspergillus oryzae type III polyketide synthase CsyB. Bioorg. Med. Chem. 2010, 18, 4542–4546. [Google Scholar] [CrossRef] [PubMed]
- Sucipto, H.; Sahner, J.H.; Prusov, E.; Wenzel, S.C.; Hartmann, R.W.; Koehnke, J.; Müller, R. In vitro reconstitution of a-pyrone ring formation in myxopyronin biosynthesis. Chem. Sci. 2015, 6, 5076–5085. [Google Scholar] [CrossRef] [PubMed]
- Krome, A.K.; Becker, T.; Kehraus, S.; Schiefer, A.; Gütschow, M.; Chaverra-Muñoz, L.; Hüttel, S.; Jansen, R.; Stadler, M.; Ehrens, A.; et al. Corallopyronin A: Antimicrobial discovery to preclinical development. Nat. Prod. Rep. 2022, 39, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Conti, L.; Altomare, C.; Bottalico, A.; Sindona, G.; Segre, A.L.; Logrieco, A. Fusapyrone and deoxyfusapyrone, two antifungal α-Pyrones From Fusarium semitectum. Nat. Toxins 1994, 2, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.-S.; You, J.; Wendt, K.; King, J.; Cichewicz, R.H. Resolving a Natural Product Cold Case: Elucidation of Fusapyrone Structure and Absolute Configuration and Demonstration of Their Fungal Biofilm Disrupting Properties. J. Org. Chem. 2023, 88, 9167–9186. [Google Scholar] [CrossRef]
- Altomare, C.; Pengue, R.; Favilla, M.; Evidente, A.; Visconti, A. Structure–Activity Relationships of Derivatives of Fusapyrone, an Antifungal Metabolite of Fusarium semitectum. J. Agric. Food Chem. 2004, 52, 2997–3001. [Google Scholar] [CrossRef]
- Lim, Y.J.; Choi, E.; Park, S.-H.; Kwon, H.-J. Genetic localization of the orevactaene/epipyrone biosynthetic gene cluster in Epicoccum nigrum. Bioorg. Med. Chem. Lett. 2020, 30, 127242. [Google Scholar] [CrossRef]
- Lee, A.J.; Cadelis, M.M.; Kim, S.H.; Swift, S.; Copp, B.R.; Villas-Boas, S.G. Epipyrone A, a Broad-Spectrum Antifungal Compound Produced by Epicoccum nigrum ICMP 19927. Molecules 2020, 25, 5997. [Google Scholar] [CrossRef]
- Uchida, R.; Imasato, R.; Yamaguchi, Y.; Masuma, R.; Shiomi, K.; Tomoda, H.; Omura, S. New Sesquicillins, Insecticidal Antibiotics Produced by Albophoma sp. FKI-1778. J. Antibiot. 2005, 58, 397–404. [Google Scholar]
- Lin, R.; Kim, H.; Hong, J.; Li, Q.-J. Biological Evaluation of Subglutinol A As a Novel Immunosuppressive Agent for Inflammation Intervention. ACS Med. Chem. Lett. 2014, 5, 485–490. [Google Scholar] [CrossRef]
- Kaifuchi, S.; Mori, M.; Nonaka, K.; Masuma, R.; Ōmura, S.; Shiomi, K. Sartorypyrone D: A new NADH-fumarate reductase inhibitor produced by Neosartorya fischeri FO-5897. J. Antibiot. 2015, 68, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Oakley, C.E.; Jenkinson, C.B.; Chiang, Y.-M.; Lee, C.-K.; Jones, C.G.; Seidler, P.M.; Nelson, H.M.; Todd, R.B.; Wang, C.C.C.; et al. A heterologous expression platform in Aspergillus nidulans for the elucidation of cryptic secondary metabolism biosynthetic gene clusters: Discovery of the Aspergillus fumigatus sartorypyrone biosynthetic pathway. Chem. Sci. 2023, 14, 11022–11032. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, K.; Kanokmedhakul, S.; Suwannatrai, R.; Soytong, K.; Prabpai, S.; Kongsaeree, P. Bioactive meroterpenoids and alkaloids from the fungus Eurotium chevalieri. Tetrahedron 2011, 67, 5461–5468. [Google Scholar] [CrossRef]
- Tsukada, K.; Shinki, S.; Kaneko, A.; Murakami, K.; Irie, K.; Murai, M.; Miyoshi, H.; Dan, S.; Kawaji, K.; Hayashi, H.; et al. Synthetic biology based construction of biological activity-related library of fungal decalin-containing diterpenoid pyrones. Nat. Commun. 2020, 11, 1830–1841. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nonaka, T.; Fujii, I. Fungal type III polyketide synthases. Nat. Prod. Rep. 2014, 31, 1306–1317. [Google Scholar] [CrossRef]
- Abe, I. Engineered Biosynthesis of Plant Polyketides: Structure-Based and Precursor-Directed Approach. Top. Curr. Chem. 2010, 297, 45–66. [Google Scholar]
- Zhou, Y.; Mirts, E.N.; Yook, S.; Waugh, M.; Martini, R.; Jin, Y.-S.; Lu, Y. Reshaping the 2-Pyrone Synthase Active Site for Chemoselective Biosynthesis of Polyketides. Angew. Chem. Int. Ed. 2023, 62, e202212440. [Google Scholar] [CrossRef]
- Kalaitzis, J.A. Discovery, Biosynthesis, and Rational Engineering of Novel Enterocin and Wailupemycin Polyketide Analogues. Methods Mol. Biol. 2013, 1055, 171–189. [Google Scholar]
- Pluskal, T.; Torrens-Spence, M.P.; Fallon, T.R.; De Abreu, A.; Shi, C.H.; Weng, J.-K. The biosynthetic origin of psychoactive kavalactones in kava. Nat. Plants 2019, 5, 867–878. [Google Scholar] [CrossRef]
- Zhou, W.; Zhuang, Y.; Bai, Y.; Bi, H.; Liu, T.; Ma, Y. Biosynthesis of phlorisovalerophenone and 4-hydroxy-6-isobutyl-2-pyrone in Escherichia coli from glucose. Microb. Cell Fact. 2016, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hoefgen, S.; Gherlone, F.; Valiante, V. Intrinsic Ability of the β-Oxidation Pathway To Produce Bioactive Styrylpyrones. Angew. Chem. Int. Ed. 2022, 61, e202206851. [Google Scholar] [CrossRef]
- Bergmann, P.; Takenberg, M.; Frank, C.; Zschätzsch, M.; Werner, A.; Berger, R.G.; Ersoy, F. Cultivation of Inonotus hispidus in Stirred Tank and Wave Bag Bioreactors to Produce the Natural Colorant Hispidin. Fermentation 2022, 8, 541. [Google Scholar] [CrossRef]
- Pogorevc, D.; Panter, F.; Schillinger, C.; Jansen, R.; Wenzel, S.C.; Müller, R. Production optimization and biosynthesis revision of corallopyronin A, a potent anti-filarial antibiotic. Metab. Eng. 2019, 55, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Agarwal, V. Biosynthesis-Guided Discovery and Engineering of α-Pyrone Natural Products from Type I Polyketide Synthases. ACS Chem. Biol. 2023, 18, 1060–1065. [Google Scholar]
- Pan, L.; Yang, L.; Huang, Y.; Liang, Y.; He, Q.; Yang, D. Combinatorial Enzymatic Synthesis of Unnatural Long-Chain β-Branch Pyrones by a Highly Promiscuous Enzyme. ACS Omega 2019, 4, 21078–21082. [Google Scholar] [CrossRef]
- Li, W.; Fan, J.; Liao, G.; Yin, W.-B.; Li, S.-M. Precursor Supply Increases the Accumulation of 4-Hydroxy-6-(4- hydroxyphenyl)-α-pyrone after NRPS–PKS Gene Expression. J. Nat. Prod. 2021, 84, 2380–2384. [Google Scholar] [CrossRef]























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedin, V.V.; Obydennov, D.L.; Usachev, S.A.; Sosnovskikh, V.Y. 4-Hydroxy-2-pyrones: Synthesis, Natural Products, and Application. Organics 2023, 4, 539-561. https://doi.org/10.3390/org4040037
Fedin VV, Obydennov DL, Usachev SA, Sosnovskikh VY. 4-Hydroxy-2-pyrones: Synthesis, Natural Products, and Application. Organics. 2023; 4(4):539-561. https://doi.org/10.3390/org4040037
Chicago/Turabian StyleFedin, Vladislav V., Dmitrii L. Obydennov, Sergei A. Usachev, and Vyacheslav Y. Sosnovskikh. 2023. "4-Hydroxy-2-pyrones: Synthesis, Natural Products, and Application" Organics 4, no. 4: 539-561. https://doi.org/10.3390/org4040037