Synthesis of 2-Substituted Benzimidazole Derivatives as a Platform for the Development of UV Filters and Radical Scavengers in Sunscreens
Abstract
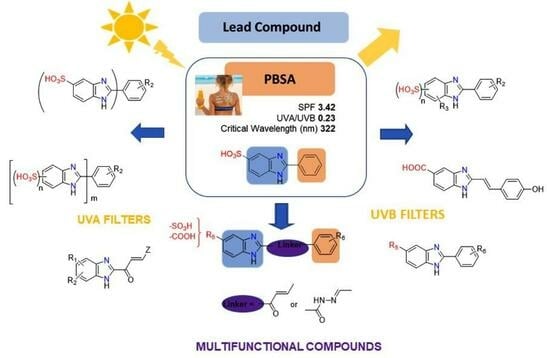
:1. Introduction
2. 2-Substituted Benzimidazoles as Photo-Protective Agents: Discovery and Development
3. Synthetic Approaches to 2-Substituted Benzimidazoles-Based UV Filters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vechtomova, Y.L.; Telegina, T.A.; Buglak, A.A.; Kritsky, M.S. UV Radiation in DNA Damage and Repair Involving. Biomedicines 2021, 9, 1564. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Hüglin, D.; Borsos, E.; Stehlin, A.; Luther, H. New UV Absorbers for Cosmetic Sunscreens—A Breakthrough for the Photoprotection of Human Skin. Chimia 2004, 58, 554–559. [Google Scholar] [CrossRef]
- Egambaram, O.P.; Pillai, S.K.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2020, 96, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.L.; Robinson, K.C.; Mao, J.; Woolf, C.J.; Fisher, D.E. Skin β-Endorphin Mediates Addiction to UV Light. Cell 2014, 157, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Revenue of the Skin Care Market Worldwide from 2015 to 2027. Statista Research Department. 2023. Available online: https://www.statista.com/forecasts/812522/sun-care-market-value-global (accessed on 27 June 2023).
- Sun Care Cosmetics Market Size, Share & Trends Analysis Report By Type (Conventional, Organic), by Distribution Channel (Specialty Stores, Online), by Product (SPF Foundation, SPF Sunscreen), And Segment Forecasts, 2023–2030. Available online: https://www.researchandmarkets.com/reports/5748305/sun-care-cosmetics-market-size-share-and-trends#rela1-5664896 (accessed on 1 February 2023).
- Manaia, E.B.; Kaminski, R.C.K.; Corrêa, M.A.; Chiavacci, L.A. Inorganic UV filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Freitas, J.V.; Lopes, N.P.; Gaspar, L.R. Photostability evaluation of five UV-filters, trans-resveratrol and beta-carotene in sunscreens. Eur. J. Pharm. Sci. 2015, 78, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Horita, K.; Sousa E Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves Da Silva, J.C.G.; Sousa Lobo, J.M. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2023, 48, 866–874. [Google Scholar] [CrossRef]
- Duarte, J.; Almeida, I.F.; Costa, M.; Da Silva, E.S.; Faria, J.L.; Sousa Lobo, J.M.; Costa, P.C.; Scalia, S. Alginate microparticles ascarriers for the UV filter 2-ethylhexyl 4-methoxycinnamate: Influence on photostability. Int. J. Cosmet. Sci. 2019, 41, 585–593. [Google Scholar] [CrossRef]
- De Oliveira, C.A.; Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and efficacy evaluation of gelatin-based nanoparticles associated with UV filters. Colloids Surf. B Biointerfaces 2016, 140, 531–537. [Google Scholar] [CrossRef]
- Scalia, S.; Tursilli, R.; Iannuccelli, V. Complexation of the sunscreen agent, 4-methylbenzylidene camphor with cyclodextrins: Effect on photostability and human stratum corneum penetration. J. Pharm. Biomed. Anal. 2007, 44, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar] [CrossRef]
- Karpkird, T.; Khunsakorn, R.; Noptheeranuphap, C.; Midpanon, S. Inclusion complexes and photostability of UV filters and curcumin with beta-cyclodextrin polymers: Effect on cross-linkers. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 37–45. [Google Scholar] [CrossRef]
- Paris, C.; Lhiaubet-Vallet, V.; Jimenez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sun-screens. J. Photochem. Photobiol. C 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef]
- Regulation (EC) № 1223/2009 of the European Parliament and of the Council: Current Consolidated Version (01/03/2022). 2022, Official Journal of the European Union. 02009R1223—EN—03.12.2020—025.001—(1–389). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R1223-20201203&rid=3 (accessed on 16 August 2023).
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2014, 37, 2–30. [Google Scholar] [CrossRef]
- Rai, R.; Shanmuga, S.C.; Srinivas, C.R. Update on photoprotection. Indian J. Dermatol. 2012, 57, 335–342. [Google Scholar] [CrossRef]
- Lebaron, P. UV filters and their impact on marine life: State of the science, data gaps, and next steps. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, L.L.R.; Harvey, K.E.; Ahmed, A.; Harvey, S.C. UV-filter pollution: Current concerns and future prospects. Environ. Monit. Assess 2021, 840, 193. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Antimicrobial potential of 1H-benzo[d]imidazole scaffold: A review. BMC Chem. 2019, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Chintakunta, R.; Meka, G. Synthesis, in silico studies and antibacterial activity of some novel 2-substituted benzimidazole derivatives. Future J. Pharm. Sci. 2020, 6, 1–6. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Wang, X.J.; Xi, M.Y.; Fu, J.H.; Zhang, F.R.; Cheng, G.F.; Yin, D.L.; You, Q.D. Synthesis, biological evaluation and SAR studies of benzimidazole derivatives as H1-antihistamine agents. Chin. Chem. Lett. 2012, 23, 707–710. [Google Scholar] [CrossRef]
- Błaszczak-Świątkiewicz, K.; Correia Almeida, D.; De Jesus Perry, M.; Mikiciuk-Olasik, E. Synthesis, anticancer activity’ and UPLC analysis of the stability of some new benzimidazole-4,7-dione derivatives. Molecules 2014, 19, 400–413. [Google Scholar] [CrossRef]
- Anichina, K.; Argirova, M.; Tzoneva, R.; Uzunova, V.; Mavrova, A.; Vuchev, D.; Popova-Daskalova, G.; Fratev, F.; Guncheva, M.; Yancheva, D. 1H-benzimidazole-2-yl hydrazones as tubulin-targeting agents: Synthesis, structural characterization, anthelmintic activity and antiproliferative activity against MCF-7 breast carcinoma cells and molecular docking studies. Chem. Biol. Interact. 2021, 345, 109540. [Google Scholar] [CrossRef]
- Argirova, M.A.; Georgieva, M.K.; Hristova-Avakumova, N.G.; Vuchev, D.I.; Popova-Daskalova, G.V.; Anichina, K.K.; Yancheva, D.Y. New 1H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity. RSC Adv. 2021, 11, 39848–39868. [Google Scholar] [CrossRef]
- Vasantha, K.; Basavaraja Swamy, G.; Rai, M.V.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426. [Google Scholar] [CrossRef]
- Veerasamy, R.; Roy, A.; Karunakaran, R.; Rajak, H. Structure–Activity Relationship Analysis of Benzimidazoles as Emerging Anti-Inflammatory Agents: An Overview. Pharmaceuticals 2021, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Merkel, E.; Wiegand, C. Light Filter. DE676103(C), 25 May 1939. [Google Scholar]
- Stevenson, C.; Davies, R.J.H. Photosensitization of guanine-specific DNA damage by 2-phenylbenzimidazole and the sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid. Chem. Res. Toxicol. 1999, 12, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Bastien, N.; Millau, J.F.; Rouabhia, M.; Davies, R.J.H.; Drouin, R. The sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid photosensitizes the formation of oxidized guanines in cellulo after UV-A or UV-B exposure. J. Investig. Dermatol. 2010, 130, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, J.J.; Bilski, P.; Chignell, C.F. Photophysical and photochemical studies of 2-phenylbenzimidazole and UVB sunscreen 2-phenylbenzimidazole-5-sulfonic acid. Photochem. Photobiol. 2002, 75, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Baron, H.; Kath, J.; Doeller, W. Kosmetisches Lichtschutzmittel. DE1282855(B), 14 November 1968. [Google Scholar]
- Hewang, U.; Stein, I.; Fechtel, U.; Casutt, M.; Faller, G.; Haertner, H. Method of Preparing 2-Arylbenzimidazole-5-sulphonic Acids. WO9315061(A2), 5 August 1993. [Google Scholar]
- Pelzer, R.; Langner, R.; Surburg, H.; Sommer, H.; Krempel, A.; Hopp, R. Utilization of Benzazols as UV-Absorbers, New Benzazoles and Process for Their Preparation. EP0669323(A1), 30 August 1995. [Google Scholar]
- Heywang, U.; Schwarz, M.; Pfluecker, F. 2-Phenylbenzimidazole Sulphonic Acids as UV-B Filters. EP1167358(A1), 2 January 2002. [Google Scholar]
- Gonzalez, A.; Pechko, A.; Anderson, G.T.; Kalafsky, R.E.; Lowenborg, M.V. Novel Esters of Aryl Benzimidazole Sulfonic Acids and Sunscreen Compositions Same. WO2005065154(A2), 21 July 2005. [Google Scholar]
- Ruehter, G.; Stenzel, W. Cosmetic for Makeup and Hair Treating Compsn.-Contains New 2-(3′-Aryl-Acryloxy)-Benzimidazole Compounds as UV Absorber. DE4107489(A1), 10 September 1992. [Google Scholar]
- Addor, F.A.S.; Barcaui, C.B.; Gomes, E.E.; Lupi, O.; Reato, C.; Hélio, M.; Miot, A. Sun-screen lotions in the dermatological prescription: Review of concepts and controversies. An. Bras. Dermatol 2022, 97, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles. Bioorg. Chem. 2020, 94, 103396. [Google Scholar] [CrossRef]
- Djuidje, E.N.; Durini, E.; Sciabica, S.; Serra, E.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System. Molecules 2020, 25, 4324. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Vertuani, S.; Manfredini, S.; Onnis, V. In-Vitro Evaluation of Antioxidant, Antiproliferative and Photo-Protective Activities of Benzimidazolehydrazone Derivatives. Pharmaceuticals 2020, 13, 68. [Google Scholar] [CrossRef]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole derivatives as multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorg. Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef]
- Onnis, V.; Demurtas, M.; Deplano, A.; Balboni, G.; Baldisserotto, A.; Manfredini, S.; Pacifico, S.; Liekens, S.; Balzarini, J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules 2016, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Serra, E. Design, Synthesis, Characterization and Investigation of Structure-Activity Relationships on Antioxidants and/or UV Filtering Properties of New Potential Sunscreen Molecule, Scientific/Disciplinary Sector (SDS) CHIM/08. Ph.D. Thesis, Università Degli Studi di Ferrara, Ferrara, Italy, 2020. [Google Scholar]
- Mamedov, V.A.; Zhukova, N.A. Recent Developments Towards Synthesis of (Het)arylbenzimidazole. Synthesis 2021, 53, 1849–1878. [Google Scholar] [CrossRef]
- Faheem, M.; Rathaur, A.; Pandey, A.; Singh, V.K.; Tiwari, A.K. A Review on the Modern Synthetic Approach of Benzimidazole Candidate. ChemistrySelect 2020, 5, 3981–3994. [Google Scholar] [CrossRef]
- Sharma, J.; Soni, P.K.; Bansal, R.; Halve, A.K. Synthetic Approaches Towards Benzimidazoles by the Reaction of o-Phenylenediamine with Aldehydes Using a Variety of Catalysts: A Review. Curr. Org. Chem. 2018, 22, 2280–2295. [Google Scholar] [CrossRef]
- Largeron, M.; Nguyen, K.M.H. Recent Advances in the Synthesis of Benzimidazole Derivatives from the Oxidative Coupling of Primary Amines. Synthesis 2018, 50, 241–253. [Google Scholar] [CrossRef]
- Alaqeel, S.I. Synthetic Approaches to Benzimidazoles from O-Phenylenediamine: A Literature Review. J. Saudi Chem. Soc. 2017, 21, 229–237. [Google Scholar] [CrossRef]
- Sayapin, V.G.; Simonov, A.M.; Kuz’menko, V.V. Reaction of Benzimidazoles with Chlorosulfonic. Acid. Chem. Heterocycl. Compd. 1970, 6, 630–632. [Google Scholar] [CrossRef]
- da Silva, M.F.; Signorini, A.M.; Vincente, J.; de Souza, B.; Priebe, J.P.; Szpoganicz, B.; Gonçalves, N.S.; Neves, A. Synthesis of substituted dipyrido[3,2-a:2′,3′-c]phenazines and a new heterocyclicdipyrido[3,2-f:2′,3′-h]quinoxalino[2,3-b]quinoxaline. Tetrahedron 2008, 64, 5410–5415. [Google Scholar] [CrossRef]
- Secci, D.; Bolasco, A.; D’Ascenzio, M.; Della Sala, F.; Yáñez, M.; Carradori, S. Conventional and microwave-assisted synthesis of benzimidazole derivatives and their in vitro inhibition of human cyclooxygenase. J. Heterocycl. Chem. 2012, 49, 1187–1195. [Google Scholar] [CrossRef]
- Göker, H.; Ölgen, S.; Ertand, R.; Akgün, H.; Özbey, S.; Kendi, E.; Topçu, G.Ü.L. Synthesis of some new benzimidazole-5-carboxylic acids. J. Heterocycl. Chem. 1995, 32, 1767–1773. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anichina, K.K.; Georgiev, N.I. Synthesis of 2-Substituted Benzimidazole Derivatives as a Platform for the Development of UV Filters and Radical Scavengers in Sunscreens. Organics 2023, 4, 524-538. https://doi.org/10.3390/org4040036
Anichina KK, Georgiev NI. Synthesis of 2-Substituted Benzimidazole Derivatives as a Platform for the Development of UV Filters and Radical Scavengers in Sunscreens. Organics. 2023; 4(4):524-538. https://doi.org/10.3390/org4040036
Chicago/Turabian StyleAnichina, Kameliya K., and Nikolai I. Georgiev. 2023. "Synthesis of 2-Substituted Benzimidazole Derivatives as a Platform for the Development of UV Filters and Radical Scavengers in Sunscreens" Organics 4, no. 4: 524-538. https://doi.org/10.3390/org4040036