Formation of OH Radicals on BiVO4–TiO2 Nanocomposite Photocatalytic Film under Visible-Light Irradiation: Roles of Photocatalytic Reduction Channels
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fluorescence Probe Detection of OH Radicals
3. Results and Discussion
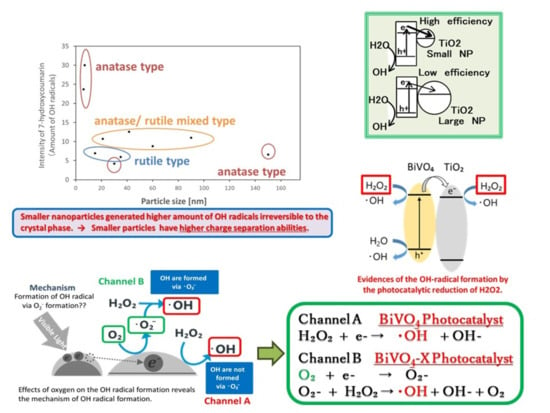
3.1. Detection of OH Radicals Formed by the BiVO4–TiO2 Photocatalyst
3.2. Effects of H2O2 Addition for OH Radical Formation by the Visible Light Irradiated BiVO4–TiO2 Photocatalyst
3.3. Influence of Oxygen on OH-Radicals Formation on the BiVO4 and the BiVO4–TiO2, Nanocomposite Photocatalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Shang, M.; Yin, W. Photocatalytic degradation of rhodamine B and phenol by solution combustion synthesized BiVO4 photocatalyst. Catal. Commun. 2010, 11, 982–986. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Huang, Y.; Lee, S.; Zhang, L. Monoclinic α-Bi2O3 photocatalyst for efficient removal of gaseous NO and HCHO under visible light irradiation. J. Alloys Compd. 2011, 509, 2044–2049. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient Photocatalytic Decomposition of Organic Contaminants over CaBi2O4 under Visible-Light Irradiation. Angew. Chem. 2004, 116, 4563–4566. [Google Scholar] [CrossRef]
- Murakami, Y.; Chatchai, P.; Nosaka, Y. Developments of the Efficient Water-splitting Electrodes under the Visible Light Irradiation. Electrochemistry 2009, 77, 44–50. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Abdi, F.; Savenije, T.J.; May, M.M.; Dam, B.; Krol, R. The Origin of Slow Carrier Transport in BiVO4 Thin Film Photoanodes: A Time-Resolved Microwave Conductivity Study. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Ziwritsch, M.; Muller, S.; Hempel, H.; Unold, T.; Abdi, F.F.; Krol, R.; Friedrich, D.; Eichberger, R. Direct Time-Resolved Observation of Carrier Trapping and Polaron Conductivity in BiVO4. ACS Energy Lett. 2016, 1, 888–894. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chem, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B Environ. 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, G.; Wei, S.; Ren, H.; Xia, A.; Luo, Y. Microwave hydrothermal synthesis and photocatalytic properties of TiO2/BiVO4 composite photocatalysts. Ceram. Int. 2013, 39, 8597–8604. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chainset, S.; Phanichphant, S.; Wetchakun, K. Efficient photocatalytic degradation of methylene blue over BiVO4/TiO2 nanocomposites. Ceram. Int. 2014, 41, 5999–6004. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Wei, Z.; Ye, S.; Dionysiou, D. Synthesis of BiVO4/P25 composites for the photocatalytic degradation of ethylene under visible light. Chem. Eng. J. 2017, 314, 443–452. [Google Scholar] [CrossRef]
- Ho-Kimura, S.; Moniz, S.J.A.; Handko, A.D.; Tang, J. Enhanced photoelectrochemical water splitting by nanostructured BiVO4–TiO2 composite electrodes. J. Mater. Chem. A 2014, 2, 3948–3953. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yang, J.; Cho, H.; Wu, J. Fabrication of an Efficient BiVO4–TiO2 Heterojunction Photoanode for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 20032–20039. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.; Grigioni, I.; Dozzi, M.; Selli, E. Sensitizing effects of BiVO4 and visible light-induced production of highly reductive electrons in the TiO2/BiVO4 heterojunction. Catal. Today 2020, 340, 19–25. [Google Scholar] [CrossRef]
- Kohtani, S.; Tomohiro, M.; Tokumura, K.; Nakagaki, R. Photooxidation reactions of polycyclic aromatic hydrocarbons over pure and Ag-loaded BiVO4 photocatalysts. Appl. Catal. B. Environ. 2005, 58, 265–272. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Generation of OH radicals and oxidation mechanism in photocatalysis of WO3 and BiVO4 powders. J. Photochem. Photobiol. A Chem. 2015, 303–304, 53–58. [Google Scholar] [CrossRef]
- Liu, G.; Wum, T.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted Degradation of Dye Pollutants. 8. Irreversible Degradation of Alizarin Red under Visible Light Radiation in Air-Equilibrated Aqueous TiO2 Dispersions. Environ. Sci. Technol. 1999, 33, 2081–2087. [Google Scholar] [CrossRef]
- Nosaka, A.Y.; Fujiwara, T.; Yagi, H.; Akutsu, H.; Nosaka, Y. Photoinduced Changes of Surface and Adsorbed Water in TiO2 Photocatalytic Systems as Studied by Solid State 1H-NMR Spectroscopy. Chem. Lett. 2002, 31, 420. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Murakami, Y.; Endo, K.; Ohta, I.; Nosaka, A.Y.; Nosaka, Y. Can OH Radicals Diffuse from the UV-Irradiated Photocatalytic TiO2 Surfaces? Laser-Induced-Fluorescence Study. J. Phys. Chem. C 2007, 111, 11339–11346. [Google Scholar] [CrossRef]
- Deguchi, S.; Shibata, N.; Takeuchi, T.; Fujiwara, Y.; Isu, N. Photocatalytic Hydrogen Production from Aqueous Solution of Various Oxidizing Sacrifice Agents. J. Jpn. Petroleum. Inst. 2010, 52, 95–100. [Google Scholar] [CrossRef]
- Louie, G.; Foley, S.; Cabilic, J.; Coffigny, H.; Taran, F.; Valleix, A.; Renault, J.P.; Pin, S. The reaction of coumarin with the OH radical revisited: Hydroxylation product analysis determined by fluorescence and chromatography. Radiat. Phys. Chem. 2005, 72, 119–124. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Quantitative detection of OH radicals for investigating the reaction mechanism of Various visible-light TiO2 photocatalysts in aqueous suspension. J. Phys. Chem. C 2013, 117, 1383–1391. [Google Scholar] [CrossRef]
- Dibbel, R.S.; Watson, D.F. Distance-Dependent Electron Transfer in Tethered Assemblies of CdS Quantum Dots and TiO2 Nanoparticles. J. Phys. Chem. C 2009, 113, 3139–3149. [Google Scholar] [CrossRef]
- Du, L.; Furube, A.; Yamamoto, K.; Hara, K.; Katoh, R.; Tachiya, M. Plasmon-Induced Charge Separation, and Recombination Dynamics in Gold-TiO2 Nanoparticle Systems: Dependence on TiO2 Particle Size. J. Phys. Chem. C 2009, 113, 6454–6462. [Google Scholar] [CrossRef]
- Liu, S.; Jaffrezic, N.; Guillard, C. Size effects in liquid-phase photo-oxidation of phenol using nanometer-sized TiO2 catalysts. Appl. Surf. Sci. 2008, 255, 2704–2709. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Evidence for H2O2 Generation during the TiO2-Assisted Photodegradation of Dyes in Aqueous Dispersions under Visible Light Illumination. J. Phys. Chem. B 1999, 103, 4862–4867. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Zhao, J. Mechanism of Photodecomposition of H2O2 on TiO2 surfaces under Visible Light Irradiation. Langmuir 2001, 17, 4118–4122. [Google Scholar] [CrossRef]
- Hayashi, T.; Nakamura, K.; Suzuki, T.; Saito, N.; Murakami, Y. OH radical formation by the photocatalytic reduction reactions of H2O2 on the surface of plasmonic excited Au-TiO2 photocatalysts. Chem. Phys. Lett. 2020, 739, 136958. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2 and OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nishikawa, M.; Saito, N.; Terashima, C.; Fujishima, A. Significance of Hydroxyl Radical in Photoinduced Oxygen Evolution in Water on Monoclinic Bismuth Vanadate. J. Phys. Chem. C 2017, 121, 25624–25631. [Google Scholar] [CrossRef]
- Shi, L.; Xu, C.; Zhang, H.; Liu, Z.; Qu, X.; Du, F. Facile fabrication of hierarchical BiVO4/TiO2 heterostructures for enhanced photocatalytic activities under visible-light irradiation. J. Mater. Sci. 2018, 53, 11329–11342. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, N.; Luo, M.; Fan, L.; Zhao, K.; Qu, J.; Guan, J.; Yuan, X. Enhancement mechanism of fiddlehead-shaped TiO2-BiVO4 type II heterojunction in SPEC towards RhB degradation and detoxification. Appl. Surf. Sci. 2019, 463, 234–243. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Furube, A.; Du, L.; Hara, K.; Kato, R.; Tachiya, M. Ultrafast Plasmon-Induced Electron Transfer from Gold Nanodots into TiO2 Nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef]
- Du, L.; Furube, A.; Hara, K.; Kato, R.; Tachiya, M. Ultrafast plasmon-induced electron injection mechanism in gold–TiO2 nanoparticle system. J. Photochem. Photobiol. C 2013, 15, 21–30. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge Carrier Dynamics at TiO2 Particles: Reactivity of Free and Trapped Holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Henderson, M.; Epling, W.S.; Peden, C.H.; Perkins, C.L. Insights into Photoexcited Electron Scavenging Processes on TiO2 Obtained from Studies of the Reaction of O2 with OH Groups Adsorbed at Electronic Defects on TiO2(110). J. Phys. Chem. B 2003, 107, 534–545. [Google Scholar] [CrossRef]
- Losada, C.M.; Johansson, J.; Brinck, T.; Jonsson, M. Mechanism of H2O2 Decomposition on Transition Metal Oxide Surfaces. J. Phys. Chem. C 2012, 116, 9533–9543. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Yu, T.; Breslin, C.B. Review—2D Graphene and Graphene-Like Materials and Their Promising Applications in the Generation of Hydrogen Peroxide. J. Electrochem. Soc. 2020, 167, 126502. [Google Scholar] [CrossRef]
- Xie, L.; Hao, J.-G.; Chen, H.-Q.; Li, Z.-X.; Ge, S.-Y.; Mi, Y.; Yang, K.; Lu, K. Recent advances of nickel hydroxide-based cocatalysts in heterogeneous photocatalysis. Catal. Commun. 2022, 162, 106371. [Google Scholar] [CrossRef]
- Wei, Y.; Hao, J.-G.; Zhang, J.-L.; Huang, W.-Y.; Ouyang, S.-B.; Yang, K.; Lu, K.-Q. Integrating Co(OH)2 nanosheet arrays on graphene for efficient noble-metal-free EY-sensitized photocatalytic H2 evolution. Dalton. Trans. 2023, 52, 13923–13929. [Google Scholar] [CrossRef]







| Name | Anatase Content (%) | Rutile Content (%) | Primary Particle Size (nm) | BET Surface Area (m2 g−1) | Supplier |
|---|---|---|---|---|---|
| ST-01 | 100 | 0 | 7 | 320 [21,22] | Ishihara Sangyo |
| F1 | 90 | 10 | 50 | 26 [23] | Showa Titanuim |
| F2 | 60 | 40 | 40 | - | Showa Titanuim |
| P25 | 70 [20] | 30 | 21 | 32 [21], 49 [22] | Degussa |
| AMT-100 | 100 | 0 | 6 | 6 [21] | Tayca |
| AMT-600 | 100 | 0 | 30 | 30 [21], 49 [22] | Tayca |
| MT-150A | 0 | 100 | 15 | 88 [22] | Tayca |
| MT-500B | 0 | 100 | 35 | 38 [23] | Tayca |
| Wako | 100 | 0 | 41.5 | 8.3 [24] | Wako Chemicals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terao, S.; Murakami, Y. Formation of OH Radicals on BiVO4–TiO2 Nanocomposite Photocatalytic Film under Visible-Light Irradiation: Roles of Photocatalytic Reduction Channels. Reactions 2024, 5, 98-110. https://doi.org/10.3390/reactions5010004
Terao S, Murakami Y. Formation of OH Radicals on BiVO4–TiO2 Nanocomposite Photocatalytic Film under Visible-Light Irradiation: Roles of Photocatalytic Reduction Channels. Reactions. 2024; 5(1):98-110. https://doi.org/10.3390/reactions5010004
Chicago/Turabian StyleTerao, Shizu, and Yoshinori Murakami. 2024. "Formation of OH Radicals on BiVO4–TiO2 Nanocomposite Photocatalytic Film under Visible-Light Irradiation: Roles of Photocatalytic Reduction Channels" Reactions 5, no. 1: 98-110. https://doi.org/10.3390/reactions5010004