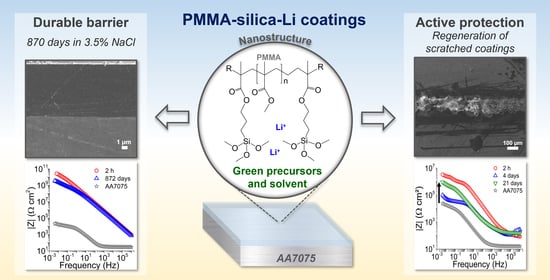
Green-High-Performance PMMA–Silica–Li Barrier Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PMMA–Silica–Li Hybrids
2.2. Deposition of the Coatings
2.3. Characterization
2.3.1. Chemical and Structural Analysis
2.3.2. Adhesion, Thickness, and Morphology of Coatings
2.3.3. Thermal Analysis
2.3.4. Electrochemical Measurements
3. Results and Discussion
3.1. Structural Properties
3.2. Thermal Stability
3.3. Electrochemical Barrier Properties
| Coating | Solvent | Substrate | Electrolyte | Thickness (µm) | ǀZlfǀ (Ωcm2) | Lifetime (Days) | Thermal Stability * (°C) | Adhesion (MPa) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PMMA–silica–Li | 2-propanol | AA7075 | 0.6 M NaCl | 10 | 1010 | >872 | 245 | 15.5 | This work |
| PMMA–silica–Ce | THF | Mild steel | 0.6 M NaCl | 26 | 109 | 354 | - | - | [29] |
| PMMA–silica | THF | AA7075 | 0.1 M NaCl | 5 | 108 | >60 | - | - | [21] |
| PMMA–silica | THF | AA7075 | 0.1 M NaCl | 1.4 | 109 | >120 | 90 | - | [30] |
| PMMA–silica | THF | AA2024 | 0.1 M NaCl | 4 | 109 | >180 | - | - | [31] |
| PMMA–silica | THF | Steel | 0.6 M NaCl | 4 | 109 | >180 | - | - | [32] |
| PMMA–silica | THF | AA7075 | 0.85 M NaCl | 5 | 109 | >216 | - | - | [33] |
| Acrylic polyol –silica | Butyl acetate | Mild steel | 0.6 M NaCl | 75 | 109 | >90 | - | - | [34] |
| Acrylic polyol –silica–ZnO | Xylene | Mild steel | 0.6 M NaCl | 75 | 109 | >30 | - | - | [35] |
| GMA–EHA–silica | THF | AA1050 | 0.1 M NaCl | 1 | 109 | 21 | ~250 | - | [36] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guinier, A. Structure of Age-Hardened Aluminium-Copper Alloys. Nature 1938, 142, 569–570. [Google Scholar] [CrossRef]
- Ringer, S.P.; Hono, K. Microstructural Evolution and Age Hardening in Aluminium Alloys: Atom Probe Field-Ion Microscopy and Transmission Electron Microscopy Studies. Mater. Charact. 2000, 44, 101–131. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: And Introduction, 9th ed.; Callister, W.D., Jr., Rethwisch, D.G., Eds.; Wiley: Hoboken, NJ, USA, 2014; Volume 4. [Google Scholar]
- Trentin, A.; de Gasparini, A.L.; Faria, F.A.; Harb, S.V.; dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Barrier Properties of High Performance PMMA-Silica Anticorrosion Coatings. Prog. Org. Coat. 2020, 138, 105398. [Google Scholar] [CrossRef]
- Echeverría, M.; Abreu, C.M.; Lau, K.; Echeverría, C.A. Viability of Epoxy-Siloxane Hybrid Coatings for Preventing Steel Corrosion. Prog. Org. Coat. 2016, 92, 29–43. [Google Scholar] [CrossRef]
- Del Angel-López, D.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Flores-Vela, A.; Andraca-Adame, J.; Dorantes-Rosales, H. Analysis of Degradation Process during the Incorporation of ZrO2:SiO2 Ceramic Nanostructures into Polyurethane Coatings for the Corrosion Protection of Carbon Steel. J. Mater. Sci. 2013, 48, 1067–1084. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of Advanced Hybrid Organic-Inorganic Nanomaterials: From Laboratory to Market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Dos Santos, F.C.; Harb, S.V.; Menu, M.-J.; Turq, V.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. On the Structure of High Performance Anticorrosive PMMA–Siloxane-Silica Hybrid Coatings. RSC Adv. 2015, 5, 106754. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Protective PMMA-Silica Coatings for Aluminum Alloys: Nanostructural Control of Elevated Thermal Stability and Anticorrosive Performance. Prog. Org. Coat. 2021, 152, 106129. [Google Scholar] [CrossRef]
- Trentin, A.; Harb, S.V.; Uvida, M.C.; Pulcinelli, S.H.; Santilli, C.V.; Marcoen, K.; Pletincx, S.; Terryn, H.; Hauffman, T.; Hammer, P. Dual Role of Lithium on the Structure and Self-Healing Ability of PMMA-Silica Coatings on AA7075 Alloy. ACS Appl. Mater. Interfaces 2019, 11, 40629–40641. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Torrico, R.F.O.; Pulcinelli, S.H.; Santilli, C.V.; Peter, H. Organic-Inorganic Hybrid Coatings for Corrosion Protection of Metallic Surfaces. In New Technologies in Protective Coatings; IntechOpen: London, UK, 2016; pp. 19–51. [Google Scholar]
- Billmeyer, F.W., Jr. Radical Chain (Addition) Polymerization. In Textbook of Polymer Science; Wiley-Interscience: Singapore, 1984; pp. 49–77. ISBN 0471031968. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. The Physics and Chemistry of Sol-Gel Processing. In Sol-Gel Science; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780080571034. [Google Scholar]
- Uvida, M.C.; Trentin, A.; Harb, S.V.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Nanostructured Poly(Methyl Methacrylate)−silica Coatings for Corrosion Protection of Reinforcing Steel. ACS Appl. Nano Mater. 2022, 5, 2603–2615. [Google Scholar]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s Solvent Selection Guide—Embedding Sustainability into Solvent Selection Starting at Medicinal Chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Guinier, G.; Fournet, C.; Walker, C.B.; Yudovitch, K.L. Small Angle Scattering of X-rays; Freeman: New York, NY, USA, 1955. [Google Scholar]
- Al Zoubi, W.; Yoon, D.K.; Kim, Y.G.; Ko, Y.G. Fabrication of Organic-Inorganic Hybrid Materials on Metal Surface for Optimizing Electrochemical Performance. J. Colloid Interface Sci. 2020, 573, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, B. A New Guinier-Porod Model. J. Appl. Crystallogr. 2010, 43, 716–719. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. Available online: htttp://srdata.nist.gov/XPS/ (accessed on 1 June 2022).
- Gandhi, J.S.; Singh, S.; Van Ooij, W.J.; Puomi, P. Evidence for Formation of Metallo-Siloxane Bonds by Comparison of Dip-Coated and Electrodeposited Silane Films. J. Adhes. Sci. Technol. 2006, 20, 1741–1768. [Google Scholar] [CrossRef]
- Hamulić, D.; Rodič, P.; Poberžnik, M.; Jereb, M.; Kovač, J.; Milošev, I. The Effect of the Methyl and Ethyl Group of the Acrylate Precursor in Hybrid Silane Coatings Used for Corrosion Protection of Aluminium Alloy 7075-T6. Coatings 2020, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Franquet, A.; Terryn, H.; Vereecken, J. IRSE Study on Effect of Thermal Curing on the Chemistry and Thickness of Organosilane Films Coated on Aluminium. Appl. Surf. Sci. 2003, 211, 259–269. [Google Scholar] [CrossRef]
- Trentin, A.; Harb, S.V.; Uvida, M.C.; Marcoen, K.; Pulcinelli, S.H.; Santilli, C.V.; Terryn, H.; Hauffman, T.; Hammer, P. Effect of Ce(III) and Ce(IV) Ions on the Structure and Active Protection of PMMA-Silica Coatings on AA7075 Alloy. Corros. Sci. 2021, 189, 109581. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Uvida, M.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. A Comparative Study on PMMA-TiO2 and PMMA-ZrO2 Protective Coatings. Prog. Org. Coat. 2019, 140, 105477. [Google Scholar] [CrossRef]
- Spirin, Y.L.; Arest-Yakubovich, A.A.; Polyakov, D.K.; Gantmakher, A.R.; Medvedev, S.S. Polymerization Catalyzed by Lithium and Lithium Alkyl. J. Polym. Sci. 1962, 58, 1181–1189. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Jacquemin, M.; Page, S.J.; Li, S.; Bertazzo, S.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. Lithium-Silicate Sol-Gel Bioactive Glass and the Effect of Lithium Precursor on Structure-Property Relationships. J. Sol-Gel Sci. Technol. 2017, 81, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Harb, S.V.; Rodrigues, M.S.; de Souza, T.A.C.; Trentin, A.; Uvida, M.C.; Pochapski, D.J.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Smart PMMA-cerium Oxide Anticorrosive Coatings Effect of Ceria Content.Pdf. Prog. Org. Coat. 2021, 161, 106548. [Google Scholar] [CrossRef]
- Visser, P.; Gonzalez-Garcia, Y.; Mol, J.M.C.; Terryn, H. Mechanism of Passive Layer Formation on AA2024-T3 from Alkaline Lithium Carbonate Solutions in the Presence of Sodium Chloride. J. Electrochem. Soc. 2018, 165, C60–C70. [Google Scholar] [CrossRef] [Green Version]
- Mosa, J.; Rosero-Navarro, N.C.; Aparicio, M. Active Corrosion Inhibition of Mild Steel by Environmentally-Friendly Ce-Doped Organic-Inorganic Sol-Gel Coatings. RSC Adv. 2016, 6, 39577–39586. [Google Scholar] [CrossRef]
- Rodič, P.; Korošec, R.C.; Kapun, B.; Mertelj, A.; Milošev, I. Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers 2020, 12, 948. [Google Scholar] [CrossRef] [Green Version]
- Rodič, P.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Milošev, I. The Effect of Copolymerisation on the Performance of Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA2024-T3. Prog. Org. Coat. 2020, 147, 105701. [Google Scholar] [CrossRef]
- Hamulić, D.; Rodič, P.; Milošev, I. The Influence of Length of Alkyl Chain on the Chemical Structure and Corrosion Resistance of Silica-Polyacrylic Hybrid Coatings on Structural Steel. Prog. Org. Coat. 2021, 150, 105982. [Google Scholar] [CrossRef]
- Milošev, I.; Hamulić, D.; Rodič, P.; Carrière, C.; Zanna, S.; Budasheva, H.; Korte, D.; Franko, M.; Mercier, D.; Seyeux, A.; et al. Siloxane Polyacrylic Sol-Gel Coatings with Alkly and Perfluoroalkyl Chains: Synthesis, Composition, Thermal Properties and Log-Term Corrosion Protection. Appl. Surf. Sci. 2022, 574, 151578. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. A Novel Coating Material That Uses Nano-Sized SiO2particles to Intensify Hydrophobicity and Corrosion Protection Properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Formulation and Characterization of Hybrid Polymeric/ZnO Nanocomposite Coatings with Remarkable Anti-Corrosion and Hydrophobic Characteristics. J. Coat. Technol. Res. 2016, 13, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Khelifa, F.; Druart, M.E.; Habibi, Y.; Bénard, F.; Leclère, P.; Olivier, M.; Dubois, P. Sol-Gel Incorporation of Silica Nanofillers for Tuning the Anti-Corrosion Protection of Acrylate-Based Coatings. Prog. Org. Coat. 2013, 76, 900–911. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M.F. “SMART” Protective Ability of Water Based Epoxy Coatings Loaded with CaCO3 Microbeads Impregnated with Corrosion Inhibitors Applied on AA2024 Substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active Protection Coatings with Layered Double Hydroxide Nanocontainers of Corrosion Inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Visser, P.; Lutz, A.; Mol, J.M.C.; Terryn, H. Study of the Formation of a Protective Layer in a Defect from Lithium-Leaching Organic Coatings. Prog. Org. Coat. 2016, 99, 80–90. [Google Scholar] [CrossRef]










| Sample | Li0 | Li05 | Li1 | Li2 | Li4 |
|---|---|---|---|---|---|
| Thickness (µm) | 12.2/8.6 | 7.8/7.1 | 6.8/7.8 | 10.0/9.8 | 8.7/8.0 |
| Adhesion (MPa) | 10.6/9.9 | 9.3/9.4 | 11.0/11.2 | 15.5/15.4 | 6.8/7.5 |
| Tmax (°C) | 226/239 | 248/241 | 258/254 | 245/244 | 240/268 |
| Residue (%) | 20.3/17.9 | 23.3/22.9 | 25.3/25.5 | 23.3/20.6 | 27.0/29.3 |
| d (nm) | 3.1/3.2 | 3.1/3.5 | 3.3/3.4 | 3.0/3.2 | 3.4/3.2 |
| RMS roughness (nm) | 0.4/0.5 | 2.4/6.5 | 2.8/5.4 | 0.6/0.8 | 2.5/2.1 |
| Sample | Li2 | Li2 Duplicate | ||||
|---|---|---|---|---|---|---|
| 2 h | 4 d | 21 d | 2 h | 4 d | 21 d | |
| χ2 | 5.8 × 10−3 | 3.3 × 10−3 | 4.6 × 10−3 | 4.4 × 10−3 | 1.9 × 10−3 | 3.1 × 10−3 |
| Rs (Ω cm2) | 73 | 114 | 168 | 86 | 100 | 167 |
| R1 (Ω cm2) | 248 (9) | 959 (16) | 812 (12) | 246 (21) | 1948 (19) | 1330 (25) |
| Q1 (nΩ−1 cm−2 sn) | 88.3 (19) | 6377 (12) | 2180 (19) | 126 (16) | 10,800 (17) | 7290 (13) |
| n1 | 0.87 (0.13) | 0.66 (0.17) | 0.79 (0.12) | 0.91 (0.19) | 0.59 (0.12) | 0.70 (0.15) |
| R2 (MΩ cm2) | 0.98 (0.20) | 0.02 (0.01) | 0.61 (0.20) | 0.25 (0.10) | 0.01 (0.004) | 0.07 (0.03) |
| Q2 (nΩ−1 cm−2 sn) | 100 (10) | 4238 (22) | 2269 (17) | 159 (12) | 2480 (39) | 753 (28) |
| n2 | 0.94 (0.12) | 0.71 (0.15) | 0.86 (0.13) | 0.94 (0.17) | 0.82 (0.19) | 0.85 (0.13) |
| R3 (MΩ cm2) | 3.3 (0.8) | 0.02 (0.05) | 0.91 (0.16) | 4.1 (0.6) | 0.02 (0.005) | 1.04 (0.11) |
| Q3 (µΩ−1 cm−2 sn) | 2.3 (0.3) | 2637 (10) | 43.8 (2.6) | 5.1 (0.5) | 1057 (18) | 1.25 (0.14) |
| n3 | 0.79 (0.12) | 0.66 (0.11) | 0.82 (0.12) | 0.76 (0.16) | 0.66 (0.11) | 0.70 (0.13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trentin, A.; Chagas, V.H.; Uvida, M.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Green-High-Performance PMMA–Silica–Li Barrier Coatings. Corros. Mater. Degrad. 2022, 3, 303-319. https://doi.org/10.3390/cmd3030018
Trentin A, Chagas VH, Uvida MC, Pulcinelli SH, Santilli CV, Hammer P. Green-High-Performance PMMA–Silica–Li Barrier Coatings. Corrosion and Materials Degradation. 2022; 3(3):303-319. https://doi.org/10.3390/cmd3030018
Chicago/Turabian StyleTrentin, Andressa, Victória Hellen Chagas, Mayara Carla Uvida, Sandra Helena Pulcinelli, Celso Valentim Santilli, and Peter Hammer. 2022. "Green-High-Performance PMMA–Silica–Li Barrier Coatings" Corrosion and Materials Degradation 3, no. 3: 303-319. https://doi.org/10.3390/cmd3030018