Advanced Steam Reforming of Bio-Oil with Carbon Capture: A Techno-Economic and CO2 Emissions Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bio-Oil Feedstock
2.2. Desulphurisation
2.3. Economic Costing
| Unit | Base Size | Base Cost (mUSD) | f | Installation Factor | Year | Ref. |
|---|---|---|---|---|---|---|
| WGS | 15.6 Mmol h−1 CO + H2 | 36.9 | 0.85 | 1 | 2001 | [37] |
| PSA | 9600 kmol h−1 throughput | 28 | 0.7 | 1.69 | 2001 | [37] |
| CO2 capture (MDEA) | 62.59 kg s−1 CO2 captured | 104.2 | 0.8 | - | 2017 | [37] |
| CO2 compression and drying | 13 MW compressor power | 17.9 | 0.67 | - | 2017 | [18] |
| High temperature three-way valve | 2 m3 s−1 | 0.1695 | 0.6 | - | 2014 | [18] |
| HDS plant | 30,000 BPD | 16 | 0.65 | - | 1991 | [31] |
2.4. Process Modelling Methodology
- Where gas volumes are given in Nm3, normal conditions are 20 °C and 1.01325 bar;
- Air is composed of 79% N2 and 21% O2;
- To ensure storage in liquid form, the bio-oil/methanol mixture is stored above its vapour pressure (around 3 bar);
- All other fluid inputs enter the system at 25 °C and 1.01325 bar;
- Reactor pressure drop is 5% of inlet pressure;
- Heat exchanger minimum approach of 10 °C.
3. Results
3.1. Process Design Basis Selection
3.1.1. C-SR and C-SR-CCS
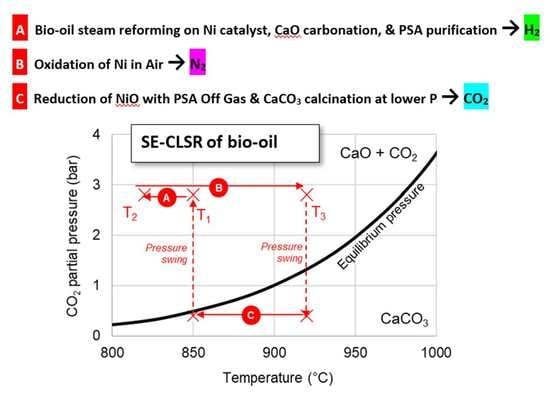
3.1.2. SE-CLSR
3.2. Process Cost Comparison
3.3. Carbon Emission Comparison
3.4. Sensitivity
3.5. Desulphurisation Impacts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an Energy Vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2020, 13, 298. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC) IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Wang, D.; Czernik, S.; Montané, D.; Mann, M.; Chornet, E. Biomass to Hydrogen via Fast Pyrolysis and Catalytic Steam Reforming of the Pyrolysis Oil or Its Fractions. Ind. Eng. Chem. Res. 1997, 36, 1507–1518. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A. Large-Scale Biohydrogen Production from Bio-Oil. Bioresour. Technol. 2010, 101, 7350–7361. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen Production from the Thermochemical Conversion of Biomass: Issues and Challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in Oxygen Carrier Development of Methane-Based Chemical-Looping Reforming: A Review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, M.; De Diego, L.F.; Abad, A.; García-Labiano, F.; Gayán, P.; Adánez, J. Catalytic Activity of Ni-Based Oxygen-Carriers for Steam Methane Reforming in Chemical-Looping Processes. Energy Fuels 2012, 26, 791–800. [Google Scholar] [CrossRef]
- Pröll, T.; Bolhàr-Nordenkampf, J.; Kolbitsch, P.; Hofbauer, H. Syngas and a Separate Nitrogen/Argon Stream via Chemical Looping Reforming—A 140 KW Pilot Plant Study. Fuel 2010, 89, 1249–1256. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-Based Oxygen Carriers in Chemical Looping Conversions: A Review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen Production by Sorption-Enhanced Chemical Looping Steam Reforming of Ethanol in an Alternating Fixed-Bed Reactor: Sorbent to Catalyst Ratio Dependencies. Energy Convers. Manag. 2018, 155, 243–252. [Google Scholar] [CrossRef]
- Pimenidou, P.; Rickett, G.; Dupont, V.; Twigg, M.V. Chemical Looping Reforming of Waste Cooking Oil in Packed Bed Reactor. Bioresour. Technol. 2010, 101, 6389–6397. [Google Scholar] [CrossRef] [Green Version]
- Spragg, J.; Mahmud, T.; Dupont, V. Hydrogen Production from Bio-Oil: A Thermodynamic Analysis of Sorption-Enhanced Chemical Looping Steam Reforming. Int. J. Hydrogen Energy 2018, 43, 22032–22045. [Google Scholar] [CrossRef]
- Brown, D.; Rowe, A.; Wild, P. Techno-Economic Comparisons of Hydrogen and Synthetic Fuel Production Using Forest Residue Feedstock. Int. J. Hydrogen Energy 2014, 39, 12551–12562. [Google Scholar] [CrossRef]
- Collodi, G.; Azzaro, G.; Ferrari, N.; Santos, S.; Brown, J.; Cotton, B.; Lodge, S. Techno-Economic Evaluation of SMR Based Standalone (Merchant) Hydrogen Plant with CCS; IEAGHG Technical Report; IEAGHG: Cheltenham, UK, 2017. [Google Scholar]
- Riva, L.; Martínez, I.; Martini, M.; Gallucci, F.; van Sint Annaland, M.; Romano, M.C. Techno-Economic Analysis of the Ca-Cu Process Integrated in Hydrogen Plants with CO2 Capture. Int. J. Hydrogen Energy 2018, 43, 15720–15738. [Google Scholar] [CrossRef]
- Romano, M.C.; Chiesa, P.; Lozza, G. Pre-Combustion CO2 Capture from Natural Gas Power Plants, with ATR and MDEA Processes. Int. J. Greenh. Gas Control. 2010, 4, 785–797. [Google Scholar] [CrossRef]
- Yang, F.; Meerman, J.C.; Faaij, A.P.C. Carbon Capture and Biomass in Industry: A Techno-Economic Analysis and Comparison of Negative Emission Options. Renew. Sustain. Energy Rev. 2021, 144, 111028. [Google Scholar] [CrossRef]
- Northern Gas Networks. H21 Leeds City Gate. Available online: https://h21.green/projects/h21-leeds-city-gate/ (accessed on 24 May 2018).
- Sutherland, F.; Duffy, L.; Ashby, D.; Legrand, P.; Jones, G.; Jude, E. Net Zero Teesside: Subsurface Evaluation of Endurance. In Proceedings of the 1st Geoscience & Engineering in Energy Transition Conference, Strasbourg, France, 16 November 2020; Volume 2020, pp. 1–5. [Google Scholar]
- CO2 Europipe Consortium. D3.1.2. Standards for CO2: CO2 Europipe Towards a Transport Infrastructure for Large-Scale CCS in Europe. 2009. Available online: http://www.co2europipe.eu/Publications/D2.2.1%20-%20CO2Europipe%20Report%20CCS%20infrastructure.pdf (accessed on 10 March 2022).
- Pimenidou, P.; Dupont, V. Characterisation of Palm Empty Fruit Bunch (PEFB) and Pinewood Bio-Oils and Kinetics of Their Thermal Degradation. Bioresour. Technol. 2012, 109, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Abdul Halim Yun, H.; Dupont, V. Thermodynamic Analysis of Methanation of Palm Empty Fruit Bunch (PEFB) Pyrolysis Oil with and without in Situ CO2 Sorption. AIMS Energy 2015, 3, 774–797. [Google Scholar] [CrossRef]
- Dupont, V.; Abdul Halim Yun, H.; White, R.; Tande, L. High Methane Conversion Efficiency by Low Temperature Steam Reforming of Biofeedstock. In Proceedings of the REGATEC 2017 4th International Conference on Renewable Energy Gas Technology, Verona, Italy, 20 April 2017; pp. 22–23. [Google Scholar]
- Garcia-Perez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Characterization of Bio-Oils in Chemical Families. Biomass Bioenergy 2007, 31, 222–242. [Google Scholar] [CrossRef]
- Darmstadt, H.; Garcia-Perez, M.; Adnot, A.; Chaala, A.; Kretschmer, D.; Roy, C. Corrosion of Metals by Bio-Oil Obtained by Vacuum Pyrolysis of Softwood Bark Residues. An X-Ray Photoelectron Spectroscopy and Auger Electron Spectroscopy Study. Energy Fuels 2004, 18, 1291–13011. [Google Scholar] [CrossRef]
- Heracleous, E. Well-to-Wheels Analysis of Hydrogen Production from Bio-Oil Reforming for Use in Internal Combustion Engines. Int. J. Hydrogen Energy 2011, 36, 11501–11511. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, T.R.; Hu, G.; Brown, R.C. Comparative Techno-Economic Analysis of Biohydrogen Production via Bio-Oil Gasification and Bio-Oil Reforming. Biomass Bioenergy 2013, 51, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Maples, R.E. Petroleum Refinery Process Economics; PennWell Corporation: Nashville, TN, USA, 2000. [Google Scholar]
- Srifa, A.; Chaiwat, W.; Pitakjakpipop, P.; Anutrasakda, W.; Faungnawakij, K. Advances in Bio-Oil Production and Upgrading Technologies. In Sustainable Bioenergy: Advances and Impacts; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2019. [Google Scholar]
- Parkash, S. Refining Processes Handbook; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2003. [Google Scholar]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.M.; et al. Net-Zero Emissions Energy Systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 978-0-13-512966-1. [Google Scholar]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis, and Design of Chemical Processes, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 53. [Google Scholar]
- Sadhukhan, J.; Ng, K.S.; Hernandez, E.M. Biorefineries and Chemical Processes: Design, Integration and Sustainability Analysis; John Wiley & Sons Ltd.: Chichester, UK, 2015. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: New York, NY, USA; London, UK, 2004. [Google Scholar]
- Hamers, H.P.; Romano, M.C.; Spallina, V.; Chiesa, P.; Gallucci, F.; Annaland, M.V.S. Comparison on Process Efficiency for CLC of Syngas Operated in Packed Bed and Fluidized Bed Reactors. Int. J. Greenh. Gas Control 2014, 28, 65–78. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable Hydrogen Production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Czernik, S.; French, R. Distributed Production of Hydrogen by Autothermal Reforming of Biomass Pyrolysis Oil. In Proceedings of the ACS National Meeting Book of Abstracts, Atlanta, GA, USA, 26–30 March 2006. [Google Scholar]
- Campanario, F.J.; Gutiérrez Ortiz, F.J. Fischer-Tropsch Biofuels Production from Syngas Obtained by Supercritical Water Reforming of the Bio-Oil Aqueous Phase. Energy Convers. Manag. 2017, 150, 599–613. [Google Scholar] [CrossRef]
- Do, T.X.; Lim, Y.I.; Yeo, H. Techno-Economic Analysis of Biooil Production Process from Palm Empty Fruit Bunches. Energy Convers. Manag. 2014, 80, 525–534. [Google Scholar] [CrossRef]
- Methanex. Methanex Posts Regional Contract Methanol Prices for North America, Europe and Asia. Available online: https://www.methanex.com/our-business/pricing (accessed on 17 September 2019).
- Song, H.; Ozkan, U.S. Economic Analysis of Hydrogen Production through a Bio-Ethanol Steam Reforming Process: Sensitivity Analyses and Cost Estimations. Int. J. Hydrogen Energy 2010, 35, 127–134. [Google Scholar] [CrossRef]
- Swanson, R.M.; Platon, A.; Satrio, J.A.; Brown, R.C. Techno-Economic Analysis of Biomass-to-Liquids Production Based on Gasification. Fuel 2010, 89, S11–S19. [Google Scholar] [CrossRef] [Green Version]
- Spallina, V.; Motamedi, G.; Gallucci, F.; van Sint Annaland, M. Techno-Economic Assessment of an Integrated High Pressure Chemical-Looping Process with Packed-Bed Reactors in Large Scale Hydrogen and Methanol Production. Int. J. Greenh. Gas Control 2019, 88, 71–84. [Google Scholar] [CrossRef]
- Nazir, S.M.; Cloete, S.; Bolland, O.; Amini, S. Techno-Economic Assessment of the Novel Gas Switching Reforming (GSR) Concept for Gas-Fired Power Production with Integrated CO2 Capture. Int. J. Hydrogen Energy 2018, 43, 8754–8769. [Google Scholar] [CrossRef] [Green Version]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic Steam Reforming of Bio-Oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Kemper, J.; Sutherland, L.; Watt, J.; Santos, S. Evaluation and Analysis of the Performance of Dehydration Units for CO2 Capture. Energy Procedia 2014, 63, 7568–7584. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.V.; Fermoso, J.; Pevida, C.; Chen, D.; Rubiera, F. Production of Fuel-Cell Grade H2 by Sorption Enhanced Steam Reforming of Acetic Acid as a Model Compound of Biomass-Derived Bio-Oil. Appl. Catal. B Environ. 2016, 184, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.M.; Román-Leshkov, Y.; Green, W.H. Investigating the Techno-Economic Trade-Offs of Hydrogen Source Using a Response Surface Model of Drop-in Biofuel Production via Bio-Oil Upgrading. Biofuels Bioprod. Biorefin. 2012, 6, 503–520. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of Thermochemical Routes for Hydrogen Production from Biomass: A Review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Marda, J.R.; DiBenedetto, J.; McKibben, S.; Evans, R.J.; Czernik, S.; French, R.J.; Dean, A.M. Non-Catalytic Partial Oxidation of Bio-Oil to Synthesis Gas for Distributed Hydrogen Production. Int. J. Hydrogen Energy 2009, 34, 8519–8534. [Google Scholar] [CrossRef]
- Spallina, V.; Pandolfo, D.; Battistella, A.; Romano, M.C.; van Sint Annaland, M.; Gallucci, F. Techno-Economic Assessment of Membrane Assisted Fluidized Bed Reactors for Pure H2 Production with CO2 Capture. Energy Convers. Manag. 2016, 120, 257–273. [Google Scholar] [CrossRef]
- Dupont, V.; Ross, A.B.; Hanley, I.; Twigg, M.V. Unmixed Steam Reforming of Methane and Sunflower Oil: A Single-Reactor Process for H2-Rich Gas. Int. J. Hydrogen Energy 2007, 32, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Marquevich, M.; Farriol, X.; Medina, F.; Montané, D. Hydrogen Production by Steam Reforming of Vegetable Oils Using Nickel-Based Catalysts. Ind. Eng. Chem. Res. 2001, 40, 4757–4766. [Google Scholar] [CrossRef]
- Aspen Plus V.10; Aspen Technology: Houston, TX, USA, 2015.
- Chattanathan, S.A.; Adhikari, S.; McVey, M.; Fasina, O. Hydrogen Production from Biogas Reforming and the Effect of H2S on CH4 Conversion. Int. J. Hydrogen Energy 2014, 39, 19905–19911. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Zhou, J.; Xiang, Y.; Zhang, F.; Lin, B.; Liu, S.; Luo, Z. Simulation and Exergetic Evaluation of Hydrogen Production from Sorption Enhanced and Conventional Steam Reforming of Acetic Acid. Int. J. Hydrogen Energy 2016, 41, 21099–21108. [Google Scholar] [CrossRef]
- Baker, E.H. 87. The Calcium Oxide-Carbon Dioxide System in the Pressure Range 1-300 Atmospheres. J. Chem. Soc. (Resumed) 1962, 87, 464–470. [Google Scholar] [CrossRef]
- Meerman, J.C.; Hamborg, E.S.; van Keulen, T.; Ramírez, A.; Turkenburg, W.C.; Faaij, A.P.C. Techno-Economic Assessment of CO2 Capture at Steam Methane Reforming Facilities Using Commercially Available Technology. Int. J. Greenh. Gas Control 2012, 9, 160–171. [Google Scholar] [CrossRef]
- Seider, W.D.; Seader, J.D.; Lwein, D.R. Product and Process Design Principles: Synthesis, Analysis and Evaluation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Schmidtsche Schack Flue Gas Convection Section in Steam Reformer Plants for Ammonia, Methanol, Hydrogen Production. Available online: https://www.schmidtsche-schack.com/products/flue-gas-convection-section-in-steam-reformer-plants/ (accessed on 25 April 2017).
- Shahabuddin, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Advances in the Thermo-Chemical Production of Hydrogen from Biomass and Residual Wastes: Summary of Recent Techno-Economic Analyses. Bioresour. Technol. 2020, 299, 122557. [Google Scholar] [CrossRef]
- The Hydrogen Council. Path to Hydrogen Competitiveness a Cost Perspective; The Hydrogen Council: Brussels, Belgium, 2020. [Google Scholar]
- Consoli, C. Bioenergy and Carbon Capture and Storage-2019 Perspective; Global CCS Institute: Melbourne, Australia, 2019. [Google Scholar]
- Alptekin, G.O.; Jayataman, A.; Schaefer, M.; Ware, M.; Hunt, J.; Dobek, F. Novel Sorbent to Clean Up Biogas for CHPs; US Department of Energy: Washington, DC, USA, 2015. [CrossRef]
- MATHESON Nanochem GuardBed for Sulfur Removal 2020. Available online: https://www.mathesongas.com/gas-equipment/ultrapurification/desulfurization/ (accessed on 9 February 2022).












| Component | Limit in CO2 |
|---|---|
| CO2 | >95 vol% |
| Ar | Total noncondensables <5 vol% |
| CH4 | |
| H2 | |
| N2 | |
| O2 | |
| H2O | No free water (<500 ppmv) |
| Utility | Requirement per m3 Bio-Oil/Methanol Feed |
|---|---|
| Power | 12.58 kWh |
| Steam | 42.79 kg |
| Fuel gas | 55.30 kWh |
| Materials | ||
| Bio-oil price | 0.2 USD kg−1 | [41,42,43] |
| Methanol price | 0.37 USD kg−1 | [44] |
| Reforming catalyst/oxygen carrier price | 20 USD kg−1 | [45,46] |
| WGS catalyst price | 60 USD kg−1 | [47] |
| CaO sorbent | 1.1 USD kg−1 | [48] |
| WHSV for steam reforming | 1 h−1 | [49] |
| GHSV for WGS | 3000 h−1 | [45,50] |
| WHSV for reforming stage of SE-CLSR | 0.8 h−1 | [51] |
| Reforming catalyst lifetime (C-SR) | 1 year | Assumed |
| Oxygen carrier lifetime (SE-CLSR) | 2 years | Assumed |
| WGS catalyst lifetime | 5 years | [52] |
| CaO sorbent lifetime | 2 years | Assumed |
| MDEA solvent a | 0.04 mUSD/year per kgCO2/s | [18] |
| Waste treatment | ||
| Waste water disposal | 0.538 USD t−1 | [53] |
| Catalyst recovery | −0.11 USD/kg | [54] |
| Utilities | ||
| Process water | 2 USD m−3 | [55] |
| Electricity (purchase) | 100 USD MWh−1 | [14] |
| Electricity (export) | 50 USD MWh−1 | [14] |
| Steam (purchase/export) | 20.9 USD MWh−1 | Calculated b |
| Natural gas | 25 USD MWh−1 | [14] |
| Cooling water | 0.4 USD m−3 | [55] |
| Other assumptions | ||
| Plant availability | 360 days per year | - |
| Conversion GBP to USD | 1.29 | [56] |
| Conversion EUR to USD | 1.13 | [56] |
| Labour cost for workers in UK industry | GBP 40,000 per year | [57] |
| Shifts worked per worker per week | 5 | - |
| Shifts per day | 3 | - |
| Weeks worked per year | 47 | - |
| C-SR | C-SR-CCS | SE-CLSR | |
|---|---|---|---|
| Reformer pressure (bar) | 30 | 30 | 20 |
| Reformer temperature (°C) | 900 | 900 | 850 |
| S/C ratio | 5 | 5 | 2 |
| NiO/C ratio | - | - | 0.7 |
| Process | Fossil-Based CO2 Emitted | Biogenic CO2 Captured | Net CO2 Emissions | CO2 Avoided |
|---|---|---|---|---|
| C-SR | 3.2 | 0 | 3.2 | - |
| C-SR-CCS | 0.46 | 8.7 | −8.2 | 11.4 |
| SE-CLSR | 1.1 | 10.6 | −9.5 | 12.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeve, J.; Grasham, O.; Mahmud, T.; Dupont, V. Advanced Steam Reforming of Bio-Oil with Carbon Capture: A Techno-Economic and CO2 Emissions Analysis. Clean Technol. 2022, 4, 309-328. https://doi.org/10.3390/cleantechnol4020018
Reeve J, Grasham O, Mahmud T, Dupont V. Advanced Steam Reforming of Bio-Oil with Carbon Capture: A Techno-Economic and CO2 Emissions Analysis. Clean Technologies. 2022; 4(2):309-328. https://doi.org/10.3390/cleantechnol4020018
Chicago/Turabian StyleReeve, Jennifer, Oliver Grasham, Tariq Mahmud, and Valerie Dupont. 2022. "Advanced Steam Reforming of Bio-Oil with Carbon Capture: A Techno-Economic and CO2 Emissions Analysis" Clean Technologies 4, no. 2: 309-328. https://doi.org/10.3390/cleantechnol4020018