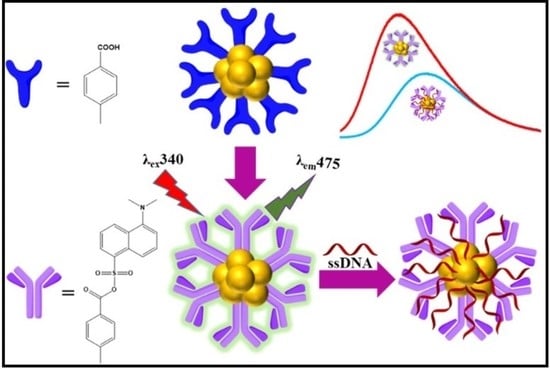
Single-Stranded DNA Recognition over Fluorescent Gold-Aryl Nanoparticles
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals
2.2. Instruments
2.3. Synthesis
2.3.1. Synthesis of AuNPs-COOH
2.3.2. Synthesis of AuNPs-DNS
2.4. Methods of ssDNA Conjugation with AuNPs-DNS
2.4.1. Freeze-Thaw Method
2.4.2. Salt-Aging Method
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Freeze-Thaw and Salt-Aging Method for ssDNA Conjugation
3.3. Assessment of Fluorescence from AuNPs-DNS/ssDNA Conjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.A.L.; Parambath, J.B.M.; Postnikov, P.S.; Guselnikova, O.; Chehimi, M.M.; Bruce, M.R.M.; Bruce, A.E.; Mohamed, A.A. Conceptual Developments of Aryldiazonium Salts as Modifiers for Gold Colloids and Surfaces. Langmuir 2021, 37, 8897–8907. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, Y.; Onidas, D.; He, L.; Jin, M.; Gazeau, F.; Pinson, J.; Mangeney, C. Surface functionalization of nanomaterials by aryl diazonium salts for biomedical sciences. Adv. Colloid Interface Sci. 2021, 294, 102479. [Google Scholar] [CrossRef] [PubMed]
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.K.; Parambath, J.B.; Gul, M.T.; Khan, A.A.; Park, Y.; Han, C.; Mohamed, A.A. Arylated gold nanostars aided SERS study of breast cancer cells. Appl. Surf. Sci. 2022, 583, 152504. [Google Scholar] [CrossRef]
- Panicker, S.; Ahmady, I.; Han, C.; Chehimi, M.; Mohamed, A. On demand release of ionic silver from gold-silver alloy nanoparticles: Fundamental antibacterial mechanisms study. Mater. Today Chem. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Sandomierski, M.; Voelkel, A. Diazonium Modification of Inorganic and Organic Fillers for the Design of Robust Composites: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 31, 1–21. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications—A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Templeton, A.C.; Hostetler, M.J.; Kraft, A.C.T.; Murray, R.W. Reactivity of Monolayer-Protected Gold Cluster Molecules: Steric Effects. J. Am. Chem. Soc. 1998, 120, 1906–1911. [Google Scholar] [CrossRef]
- Shewchuk, D.M.; McDermott, M.T. Comparison of Diazonium Salt Derived and Thiol Derived Nitrobenzene Layers on Gold. Langmuir 2009, 25, 4556–4563. [Google Scholar] [CrossRef]
- Civit, L.; Fragoso, A.; O’Sullivan, C.K. Thermal stability of diazonium derived and thiol-derived layers on gold for application in genosensors. Electrochem. Commun. 2010, 12, 1045–1048. [Google Scholar] [CrossRef]
- Laurentius, L.; Stoyanov, S.R.; Gusarov, S.; Kovalenko, A.; Du, R.; Lopinski, G.P.; McDermott, M.T. Diazonium-Derived Aryl Films on Gold Nanoparticles: Evidence for a Carbon–Gold Covalent Bond. ACS Nano 2011, 5, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Bartzatt, R. Dansylation of aromatic, aliphatic, and medicinal carboxylic acid compounds in 1 M Na2CO3 buffer. Anal. Chim. Acta 2003, 488, 203–209. [Google Scholar] [CrossRef]
- Bartzatt, R. Dansylation of hydroxyl and carboxylic acid functional groups. J. Biochem. Biophys. Methods 2001, 47, 189–195. [Google Scholar] [CrossRef]
- Gray, W.R. [8] End-group analysis using dansyl chloride. Methods Enzymol. 1972, 25, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Holmes-Farley, S.R.; Whitesides, G.M. Fluorescence properties of dansyl groups covalently bonded to the surface of oxidatively functionalized low-density polyethylene film. Langmuir 1986, 2, 266–281. [Google Scholar] [CrossRef]
- Murgia, S.; Falchi, A.M.; Meli, V.; Schillén, K.; Lippolis, V.; Monduzzi, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; Bizzarri, R.; et al. Cubosome formulations stabilized by a dansyl-conjugated block copolymer for possible nanomedicine applications. Colloids Surf. B: Biointerfaces 2015, 129, 87–94. [Google Scholar] [CrossRef]
- Szczepańska, E.; Synak, A.; Bojarski, P.; Niedziałkowski, P.; Wcisło, A.; Ossowski, T.; Grobelna, B. Dansyl-Labelled Ag@SiO2 Core-Shell Nanostructures—Synthesis, Characterization, and Metal-Enhanced Fluorescence. Materials 2020, 13, 5168. [Google Scholar] [CrossRef]
- Shibu, E.S.; Muhammed, M.A.H.; Kimura, K.; Pradeep, T. Fluorescent superlattices of gold nanoparticles: A new class of functional materials. Nano Res. 2009, 2, 220–234. [Google Scholar] [CrossRef]
- Tharmaraj, V.; Pitchumani, K. A highly selective ratiometric fluorescent chemosensor for Cu(ii) based on dansyl-functionalized thiol stabilized silver nanoparticles. J. Mater. Chem. B 2013, 1, 1962–1967. [Google Scholar] [CrossRef]
- Hameed, M.; Panicker, S.; Abdallah, S.H.; Khan, A.A.; Han, C.; Chehimi, M.M.; Mohamed, A.A. Protein-Coated Aryl Modified Gold Nanoparticles for Cellular Uptake Study by Osteosarcoma Cancer Cells. Langmuir 2020, 36, 11765–11775. [Google Scholar] [CrossRef]
- Panicker, S.; Ahmady, I.M.; Almehdi, A.M.; Workie, B.; Sahle-Demessie, E.; Han, C.; Chehimi, M.M.; Mohamed, A.A. Gold-Aryl nanoparticles coated with polyelectrolytes for adsorption and protection of DNA against nuclease degradation. Appl. Organomet. Chem. 2019, 33, e4803. [Google Scholar] [CrossRef]
- Bosak, A.; Saraf, N.; Willenberg, A.; Kwan, M.W.C.; Alto, B.W.; Jackson, G.W.; Batchelor, R.H.; Nguyen-Huu, T.D.; Sankarapani, V.; Parks, G.D.; et al. Aptamer–gold nanoparticle conjugates for the colorimetric detection of arboviruses and vector mosquito species. RSC Adv. 2019, 9, 23752–23763. [Google Scholar] [CrossRef] [PubMed]
- Saraf, N.; Bosak, A.; Willenberg, A.; Das, S.; Willenberg, B.J.; Seal, S. Colorimetric detection of epinephrine using an optimized paper-based aptasensor. RSC Adv. 2017, 7, 49133–49143. [Google Scholar] [CrossRef]
- Saraf, N.; Villegas, M.; Willenberg, B.J.; Seal, S. Multiplex Viral Detection Platform Based on a Aptamers-Integrated Microfluidic Channel. ACS Omega 2019, 4, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, I.M.; Hameed, M.K.; Almehdi, A.M.; Arooj, M.; Workie, B.; Sahle-Demessie, E.; Han, C.; Mohamed, A.A. Green and cytocompatible carboxyl modified gold–lysozyme nanoantibacterial for combating multidrug-resistant superbugs. Biomater. Sci. 2019, 7, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.-J.; Oh, S.-H.; Komathi, S.; Gopalan, A.I.; Lee, K.P.; Choi, S.-H. One-step modification of various electrode surfaces using diazonium salt compounds and the application of this technology to electrochemical DNA (E-DNA) sensors. Electrochim. Acta 2012, 76, 394–403. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Wang, X.; Zhu, J.; Zhang, R.; He, P.; Fang, Y. The application of β-cyclodextrin derivative functionalized aligned carbon nanotubes for electrochemically DNA sensing via host–guest recognition. Anal. Chim. Acta 2011, 689, 39–46. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine*. In Spherical Nucleic Acids; Jenny Stanford Publishing: Singapore, 2020; pp. 55–90. [Google Scholar] [CrossRef]
- Cutler, J.I.; Auyeung, E.; Mirkin, C.A. Spherical Nucleic Acids. J. Am. Chem. Soc. 2012, 134, 1376–1391. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Freezing Directed Construction of Bio/Nano Interfaces: Reagentless Conjugation, Denser Spherical Nucleic Acids, and Better Nanoflares. J. Am. Chem. Soc. 2017, 139, 9471–9474. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Freezing-Driven DNA Adsorption on Gold Nanoparticles: Tolerating Extremely Low Salt Concentration but Requiring High DNA Concentration. Langmuir 2019, 35, 6476–6482. [Google Scholar] [CrossRef]
- Storhoff, J.J.; Elghanian, R.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L. One-Pot Colorimetric Differentiation of Polynucleotides with Single Base Imperfections Using Gold Nanoparticle Probes. J. Am. Chem. Soc. 1998, 120, 1959–1964. [Google Scholar] [CrossRef]
- Hurst, S.J.; Lytton-Jean, A.K.R.; Mirkin, C.A. Maximizing DNA Loading on a Range of Gold Nanoparticle Sizes. Anal. Chem. 2006, 78, 8313–8318. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M.; Mirkin, C.A.; Mucic, R.C.; Reynolds, R.A.; Letsinger, R.L.; Elghanian, R.; Viswanadham, G. A Fluorescence-Based Method for Determining the Surface Coverage and Hybridization Efficiency of Thiol-Capped Oligonucleotides Bound to Gold Thin Films and Nanoparticles. Anal. Chem. 2000, 72, 5535–5541. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Optical Properties and Ultrafast Dynamics of Metallic Nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Alluhaybi, H.; Ghoshal, S.; Alsobhi, B.; Shamsuri, W.W. Visible photoluminescence from gold nanoparticles: A basic insight. Optik 2019, 192, 162936. [Google Scholar] [CrossRef]
- Ahmad, A.A.L.; Panicker, S.; Chehimi, M.M.; Monge, M.; Lopez-De-Luzuriaga, J.M.; Mohamed, A.A.; Bruce, A.E.; Bruce, M.R.M. Synthesis of water-soluble gold–aryl nanoparticles with distinct catalytic performance in the reduction of the environmental pollutant 4-nitrophenol. Catal. Sci. Technol. 2019, 9, 6059–6071. [Google Scholar] [CrossRef]
- Harper, B.; Sinche, F.; Wu, R.H.; Gowrishankar, M.; Marquart, G.; Mackiewicz, M.; Harper, S.L. The Impact of Surface Ligands and Synthesis Method on the Toxicity of Glutathione-Coated Gold Nanoparticles. Nanomaterials 2014, 4, 355–371. [Google Scholar] [CrossRef]
- Karabacak, M.; Cinar, M.; Kurt, M.; Poiyamozhi, A.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-Raman, UV and NMR) first order hyperpolarizability and HOMO–LUMO analysis of dansyl chloride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 234–244. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristics Group Frequencies; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Arivazhagan, M.; Prabhakaran, S.; Gayathri, R. Molecular structure, vibrational spectroscopic, first hyperpolarizability, NBO and HOMO, LUMO studies of P-Iodobenzene sulfonyl chloride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 332–339. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Sirimuthu, N.M.S. Surface-Enhanced Raman Spectroscopy (SERS) for Sub-Micromolar Detection of DNA/RNA Mononucleotides. J. Am. Chem. Soc. 2006, 128, 15580–15581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Servos, M.R.; Liu, J. Instantaneous and Quantitative Functionalization of Gold Nanoparticles with Thiolated DNA Using a pH-Assisted and Surfactant-Free Route. J. Am. Chem. Soc. 2012, 134, 7266–7269. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lou, X.; Wang, L.; Ding, X.; Yu, H.; Xiao, Y. Rapid, Surfactant-Free, and Quantitative Functionalization of Gold Nanoparticles with Thiolated DNA under Physiological pH and Its Application in Molecular Beacon-Based Biosensor. ACS Appl. Mater. Interfaces 2016, 8, 27298–27304. [Google Scholar] [CrossRef]
- Beha, M.J.; Ryu, J.S.; Kim, Y.S.; Chung, H.J. Delivery of antisense oligonucleotides using multi-layer coated gold nanoparticles to methicillin-resistant S. aureus for combinatorial treatment. Mater. Sci. Eng. C 2021, 126, 112167. [Google Scholar] [CrossRef] [PubMed]
- Hakimian, F.; Ghourchian, H. Simple and rapid method for synthesis of porous gold nanoparticles and its application in improving DNA loading capacity. Mater. Sci. Eng. C 2019, 103, 109795. [Google Scholar] [CrossRef]
- Javier, D.J.; Nitin, N.; Levy, M.; Ellington, A.; Richards-Kortum, R. Aptamer-Targeted Gold Nanoparticles as Molecular-Specific Contrast Agents for Reflectance Imaging. Bioconj. Chem. 2008, 19, 1309–1312. [Google Scholar] [CrossRef]
- Chang, T.-L.; Tsai, C.-Y.; Sun, C.-C.; Uppala, R.; Chen, C.-C.; Lin, C.-H.; Chen, P.-H. Electrical detection of DNA using gold and magnetic nanoparticles and bio bar-code DNA between nanogap electrodes. Microelectron. Eng. 2006, 83, 1630–1633. [Google Scholar] [CrossRef]
- Lapiene, V.; Kukolka, F.; Kiko, K.; Arndt, A.; Niemeyer, C.M. Conjugation of Fluorescent Proteins with DNA Oligonucleotides. Bioconj. Chem. 2010, 21, 921–927. [Google Scholar] [CrossRef]
- Nazarenko, I.; Pires, R.; Lowe, B.; Obaidy, M.; Rashtchian, A. Effect of primary and secondary structure of oligodeoxyribonucleotides on the fluorescent properties of conjugated dyes. Nucleic Acids Res. 2002, 30, 2089–2195. [Google Scholar] [CrossRef]
- Parambath, J.B.M.; Kanan, S.M.; Mohamed, A.A. Tryptophan capped gold-aryl nanoparticles for energy transfer study with SARS-CoV-2 spike proteins. Soft Mater. 2022, 16, 1–9. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mukherjee, S. Effects of protecting groups on luminescent metal nanoclusters: Spectroscopic signatures and applications. Chem. Commun. 2021, 58, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Lee, S.; Chen, X. The design and application of fluorophore–gold nanoparticle activatable probes. Phys. Chem. Chem. Phys. 2011, 13, 9929–9941. [Google Scholar] [CrossRef] [PubMed]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parambath, J.B.M.; Kanu, G.A.; Abu Odeh, R.O.; Kim, S.; Han, C.; Mohamed, A.A. Single-Stranded DNA Recognition over Fluorescent Gold-Aryl Nanoparticles. Colloids Interfaces 2022, 6, 42. https://doi.org/10.3390/colloids6030042
Parambath JBM, Kanu GA, Abu Odeh RO, Kim S, Han C, Mohamed AA. Single-Stranded DNA Recognition over Fluorescent Gold-Aryl Nanoparticles. Colloids and Interfaces. 2022; 6(3):42. https://doi.org/10.3390/colloids6030042
Chicago/Turabian StyleParambath, Javad B. M., Gayathri A. Kanu, Raed O. Abu Odeh, Sanghyeon Kim, Changseok Han, and Ahmed A. Mohamed. 2022. "Single-Stranded DNA Recognition over Fluorescent Gold-Aryl Nanoparticles" Colloids and Interfaces 6, no. 3: 42. https://doi.org/10.3390/colloids6030042