1. Introduction
Pollen food allergy syndrome (PFAS) is an allergic reaction to specific foods in persons previously sensitised to pollen [
1]. It is due to an allergic cross-reaction that develops between pollen allergens and allergens in foods, such as fresh fruit and vegetables. Since the prevalence of PFAS may differ from study to study, reliable data are not yet available. The prevalence of PFAS is also often expressed as a percentage in relation to another disorder (e.g., 20–70% of patients allergic to pollen have PFAS) [
2]. Indeed, prevalence studies often spring from patients presenting with allergic rhinitis or sensitised to aeroallergens associated with PFAS. Symptoms of PFAS include itchy mouth and sore throat or swelling of the lips, mouth, tongue and throat. Sometimes, itchy ears and hives around the mouth are reported [
3]. The diagnosis of PFAS is made after taking a patient’s medical history and, in some cases, conducting skin tests and oral food tests with raw fruit or vegetables [
2].
Plant-based foods are the most common cause of allergy in adults. In Mediterranean regions and especially in Italy, allergy to cypress pollen is quite common. Sensitisation is more significant in central Italy, where the reported average prevalence is 62.9% [
4].
Cupressus arizonica allergen (Cup a 1) is a major cypress allergen, used as a sensitisation marker for the Cupressaceae family [
5].
Peaches and other fruits, such as pear, apricot, strawberry, plum, apple and cherry, belong to the Rosaceae family and are among the foods most often involved in allergic reactions affecting adults and adolescents. A European study ranked peach as the most frequent cause of fruit sensitisation at 7.9% [
6]. As an allergen source, peach contains
Prunus persica (Pru p), classified in Pru p 1 (
Pathogenesis-related class 10, PR-10) and Pru p 3 (Lipid Transfer Protein, LTP). Pru p 1 is mainly found in the pulp and is thermolabile and gastrolabile, usually only causing oral allergy syndrome (OAS), while Pru p 3 is thermostable and gastrostable, triggering allergic reactions that may be serious, even with cooked foods.
A group of allergy specialists in the US reported through a specific questionnaire that the prevalence of food allergy in patients allergic to pollen was up to 5% in children and 8% in adults. In other studies, the prevalence of PFAS varied from 4.7% to 20% in children and 13% to 58% in adults [
2]. Geographical location is crucial for the assessment of the epidemiology of this phenomenon, as every region has specific aeroallergens. For example, the prevalence of PFAS is much higher in northern Europe due to the abundance of birch trees and the frequency of birch pollen allergy [
7]. It is estimated that PFAS affects 50–90% of patients allergic to birch pollen. In Mediterranean countries, there are no birch trees, and the prevalence of PFAS is lower [
8].
It is estimated that more than 30–60% of Europeans with food allergies are also allergic to pollen [
9]. PFAS occurs in persons allergic to pollen, most of whom suffer from allergic oculorhinitis or allergic asthma. It is a type 1 hypersensitivity reaction mediated by IgEs (induced by sensitisation to pollen), which cross-react with certain food allergens. Cross-reactivity develops because the pollen and the food allergens have common epitopes. This means that pollen-specific IgEs also recognise and react to food allergens with the same epitopes. Cross-reactivity in PFAS involves class 2 food allergens.
PFAS should be suspected in patients diagnosed with pollen allergy who manifest oral symptoms after eating specific foods. Various studies suggest that the easiest and safest way to obtain a diagnosis of PFAS is to test for specific IgEs in serum [
10].
In recent years, there has been growing interest in a family of plant-derived allergens known as gibberellin-regulated proteins (GRPs), involved in pollen/fruit cross-reactivity. GRPs have a structure similar to diterpenes of 19–20 carbon atoms, and these atoms are grouped in four or five rings. Widespread in plants, stable up to 90 °C and resistant to enzymatic digestion, GRPs are glycoproteins with an antimicrobial function [
11]. Being thermostable, GRPs can safely pass the stomach barrier going into the intestine and cause typical systemic reactions (since, usually, GRP allergy causes severe and very characteristic symptoms, such as laryngeal edema and angioedema). They include Pru p 7 (peamaclein), a protein detected about 10 years ago from allergic reactions to fruit of the Rosaceae family in patients who did not show positivity to any allergenic components known at the time. Only a few other members of the gibberellin-regulated protein (GRP) family have been identified as allergens since Pru p 7 was characterized as an allergen in 2013, despite the fact that these plant proteins are ubiquitous and share conserved aminoacid sequences. Inomata et al. published a paper in 2016 describing some clinical symptoms associated with Pru p7 sensitisation, which led to the first mention of the GRP allergen family.
Peamaclein is equally found in the peel and pulp of the fruit and rarely causes allergic reactions in children. Cross-reactivity was also observed between cypmaclein (GRP of cypress) and those of peach (Pru p 7), pomegranate (Pun g 7), apricot (Pru m 7) and orange (Cit s 7) [
11].
Moreover, in 2018, an allergen derived from cypress pollen (Cupressus sempervirens), previously known as BP14 since 2010 [
12], was demonstrated to belong to the GRP family [
13].
Cupressus sempervirens,
Juniperus ashei, and
Cryptomeria japonica were the only pollens that expressed allergenic GRP in greater abundance than
Hesperocyparis arizonica (formerly
Cupressus arizonica) [
14]. It has been demonstrated that BP14 and Pru p 7 are cross-reactive. Consequently, BP14 could serve as the missing link to explain the peach and cypress pollen allergies (PFAS) described in 2006 [
10,
15].
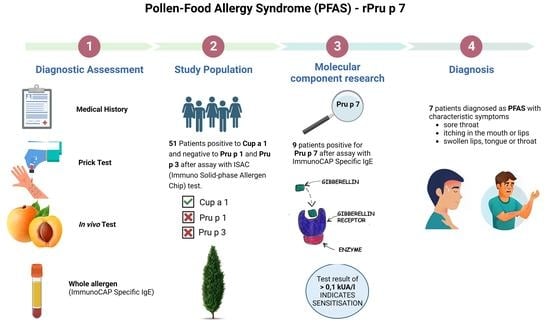
The aim of the present study was to evaluate the role of Pru p 7 in patients suspected to have PFAS, who show clinical symptoms, positivity for Cup a 1 and negativity for Pru p 1 and Pru p 3.
2. Materials and Methods
A total of 51 patients (median and interquartile range, 31 (21–42) years; 20 men and 31 women), referred to the respiratory diseases and allergology units of Siena University Hospital between May 2020 and March 2023, were enrolled retrospectively. All of them underwent allergy consultation due to the clinical history and IgE evaluation for Cup a 1, Pru p 1 and Pru p 3 by immuno solid-phase allergen chip (ISAC). The inclusion criteria were the positivity to Cup a 1 and negativity to Pru p 1 and Pru p 3 evaluated through ISAC test.
ImmunoCAP ISAC
® is a miniaturized immunoassay platform that allows for a multiplex semiquantitative measurement of IgE antibodies specific for 112 allergen molecular components in only 20 µL of serum or plasma. In the two-step assay, IgE antibodies from patient serum bind immobilized allergen components, and allergen-bound IgE antibodies are detected with a fluorescence-labelled anti-IgE antibody [
16]. Values greater than 0.3 ISU (ISAC standard unit) were considered positive. The patients included in the present study showed positivity for Cup a 1 and negativity for Pru p 1 and Pru p 3.
The Pru p 7 assay was performed by the ImmunoCAP Phadia [
17] method in patients who tested positive for Cup a 1 and simultaneously negative for Pru p 1 and Pru p 3 by ISAC. The ImmunoCAP Phadia method reacts the antigen and allergen of interest, covalently coupled to ImmunoCAP, and enzyme-labelled antibodies against IgE. Fluorescence is measured in the eluate, and values greater than 0.1 kUA/l are considered positive.
PFAS diagnosis was based on clinical signs and symptoms (including itchy mouth and ears, sore throat or swelling of the lips, mouth, tongue and throat and hives around the mouth), skin prick tests, and specific IgE testing.
Demographic, clinical and laboratory data were obtained from a digital database in order to conduct statistical analysis on the 51 patients in whom anti-Pru p 7 IgE was assessed.
The research was carried out in compliance with the Declaration of Helsinki. This study was approved by the local ethical committee (Markerlung 17431).
Statistical Analysis
Data were expressed as a mean ± standard deviation. A chi-squared test was used to compare differences in proportions. A Mann Whitney test was performed for multiple comparisons.
A Receiver Operating Characteristics (ROC) curve was employed to analyse the diagnostic performance of Pru p 7 in identifying PFAS patients and to select the best cut-off threshold with a high sensitivity and specificity. Spearman test correlation was used to evaluate the strength and direction of association between two ranked nonparametric variables.
A p-value < 0.05 was considered statistically significant. Data were analysed using GraphPad Prism 9.5.1 software.
3. Results
The serum of 51 patients was tested for sensitisation to Pru p 7 by the ImmunoCAP Phadia method, and 9 patients (17.65%) were found positive. Seven out of nine (78%) Pru p 7-positive patients showed simultaneous positivity to Cryptomeria japonica (Cry j 1), while six of them (85.7%) did to Phleum pratense (Phl p 4) and five of them (83.3%) were positive for Cynodon dactylon (Cyn d 1). Thirty out of forty-two (71%) Pru p 7-negative patients showed simultaneous positivity to Cry j 1 and Phl p 4, while twenty of them (67%) were positive for Cyn d 1 and Dermatophagoides pteronyssinus (Der p2).
Demographic and immunological data are reported in
Table 1.
Seven out of nine Pru p 7-positive patients (77.78%) were diagnosed with PFAS; these patients showed sensitisation to cypress pollen and typical symptoms of OAS after ingestion of peaches. In particular, 3/7 showed itchiness and hives around the mouth, while 4/7 showed swelling of the lips and throat and itchiness. A significant association between Pru p 7 positivity and diagnosis of PFAS was found (p < 0.0001).
An area under the receiver operating characteristic (AUROC) curve (
Figure 1) of 99.51% made it possible to distinguish PFAS and non-PFAS patients on the basis of Pru p 7 values. The best cut-off value was 0.16 kUA/l, which gave an 85.7% sensitivity and 97.73% specificity. A direct correlation was identified between the values of Cup a 1 and Pru p 7 (r = 0.357,
p = 0.010).
4. Discussion
In our study, sensitisation to Pru p 7 was evaluated in 51 patients who tested positive to cypress pollen. Specifically, all the selected patients were positive to Cup a 1 and negative to the most common peach allergens that cause allergic manifestations (Pru p 1 and Pru p 3). This criterion was used to exclude patients who had developed allergic reactions caused by allergens other than Pru p 7. An assay of the Pru p 7 allergen by the ImmunoCAP method was not included in the diagnostic routine until 2018. A total of 9 out of our 51 (17.65%) patients tested positive to Pru p 7, and 7/9 (77.78%) were diagnosed with PFAS.
The present study evaluated Pru p 7 sensitisation as a possible marker of pollen-food reactions in patients sensitised to cypress pollen. In central Italy and especially in Tuscany, cypress pollen allergy is widespread because there are many cypress trees [
18].
The Pru p 7 allergen was discovered in Italy by Tuppo et al. (2012) [
19]. They observed that some patients developed severe allergic reactions after eating peaches but tested negative to native Pru p 3 by ISAC103 [
19]. On further investigation, two of these patients tested negative by ImmunoCAP using recombinant Pru p 3. However, both patients tested positive to the skin prick test with commercial peach extract. Our results were in line with Tuppo et al., though we analysed both Pru p 1 and Pru p 3 negativity with an ISAC test.
These observations suggested that peach extract could contain another protein (different from Pru p 3) that could trigger a severe allergic reaction to peach. The authors then discovered Pru p 7 and defined its principal molecular and immunological features. Four allergenic components had previously been identified in peaches (Pru p 1, Pru p2, Pru p 3, Pru p4), and in the Mediterranean regions, Pru p 3 was the main cause of severe allergic reactions.
Inomata et al. (2014) found that the Pru p 7 allergen was an excellent marker of the risk of developing severe allergic reactions in patients allergic to peach [
20].
Sensitisation to Pru p 7 was linked to cypress pollen allergy by identifying PFAS in which allergic reactions developed after peach ingestion in patients with primary sensitisation to cypress pollen. Structurally similar to LTP, Pru p 7 is usually associated with severe allergic reactions and even anaphylaxis. Since Pru p 7 is stable to heat and digestion, even cooked peaches may cause allergic reactions in persons sensitised to Pru p 7.
A further study by Tuppo et al. showed that Pru p 7 is more resistant to simulated gastrointestinal digestion than Pru p 3 [
19]. Moreover, when peamaclein is denatured, it is sensitive to intestinal proteases but still resistant to simulated gastric digestion. By contrast, denatured Pru p 3 is sensitive to gastric and intestinal proteases. Like Pru p 3, Pru p 7 maintains its secondary structure up to 90 °C but is denatured at 100–120 °C. This influences its allergenic properties, since the protein partially loses some IgE-binding epitopes. These results suggest that persons allergic to peamaclein could eat peaches cooked for at least 10 min at 100 °C. The cooked and denatured protein could be a hypoallergenic compound useful for designing a vaccine for allergen-specific immunotherapy. According to the literature, the best method to evaluate cross-reactivity is to assay IgE specific to antigens in patients’ serum.
Few studies on the association between Pru p 7 and cypress pollen have been published in the literature. The following are worth noting:
Klingebiel et al. is a study on 316 patients with peach allergy, 171 of whom proved to be sensitised to Pru p 7. Sensitisation was associated with severe allergic reactions and the involvement of co-factors. All 171 patients except one also proved to be sensitised to cypress pollen [
21]. Possible cross-reactivity between Pru p 7 and cypress pollen was investigated in IgE antibody competition experiments: in the sera analysed, cypress pollen extract inhibited 80–100% of IgEs binding Pru p 7, whereas Pru p 7 only partly blocked the binding of IgEs to cypress pollen. This experiment and the correlation between sensitisation to Pru p 7 and exposure to cypress pollen suggest that cypress pollen acts as a primary sensitiser, activating IgEs that also cross-react with peach Pru p 7.
A study in 2021 by Biagioni et al. on a paediatric population proposed some criteria to facilitate diagnosis of allergy to Pru p 7, also considering the correlation with cypress pollen allergy [
22]. The patients to test for sensitisation to Pru p 7 were selected on the basis of the following features: clinical history of systemic allergic reaction after eating food, skin prick test positive to cypress pollen extract and peach skin extract enriched with Pru p 3 and negativity for IgE anti-Pru p 3.
These criteria proved very accurate for identifying patients sensitised to peamaclein; indeed, 100% of the patients selected showed IgEs specific for Pru p 7. The conclusion of the study was that peamaclein allergy should be suspected in patients with all these characteristics.
Asero et al. (2021) investigated the frequency of sensitisation to peamaclein in patients with cypress pollen allergy in Italy [
23]. Out of 853 patients with cypress pollen allergy, only 24 showed the specific features suggesting mono-sensitisation to Pru p 7: sensitisation to cypress pollen, skin prick test positive to commercial peach extract and Pru p 3 negativity by ImmunoCAP. Of these, only 10 patients showed IgEs specific for Pru p 7. After assessing a large population of patients sensitised to cypress pollen, the study concluded that the frequency of sensitisation to Pru p 7 is low in Italy, whereas sensitisation to Pru p 3 is much more frequent. The authors also found that only one patient sensitised to peamaclein reported a severe allergic reaction (anaphylaxis).
Analogously, we confirmed a low sensitisation to Pru p 7 in an Italian cohort, despite the small number of patients included in our study.
This finding is in contrast with other studies in which Pru p 7 was identified as a marker of possible severe allergic reactions. This difference could depend, among other things, on the selection criteria used in our study and by Asero et al., where testing for sensitisation to peach in a large population of patients allergic to cypress pollen, irrespective of any clinical history of food allergy, can give completely different results from those in patients selected on the basis of fruit allergy. In the study, it is suggested that this sensitisation may be symptomatic or associated with oral allergic syndrome.